Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D01GYT
|
||||
| Former ID |
DAP000767
|
||||
| Drug Name |
Fosfomycin
|
||||
| Synonyms |
FCM; Phosphomycin; Phosphonemycin; Phosphonomycin; Priomicina; Veramina; Disodium fosfomycin; Disodium phosphonomycin; Fosfocina disodium salt; Fosfomycin disodium; Fosfomycin disodium salt; Fosfomycin sodium; Fosfomycin sodium salt; Fosmicin S; Phosphomycin disodium salt; Phosphonomycin disodium salt; Phosphonomycin sodium; Sodium fosfomycin; MK 955; FOM-Na; Fosfomycin sodium (JP15); Fosmicin S (TN); Fosfomycin (USAN/INN); L-cis-1,2-epoxypropylphosphonic acid; Disodium (1R,2S)-(1,2-epoxypropyl)phosphonate; Disodium[(2r,3s)-3-methyloxiran-2-yl]phosphonate; [(2R,3S)-3-methyloxiran-2-yl]phosphonic acid; Phosphonic acid, (3-methyloxiranyl)-, disodium salt, (2R-cis)-(9CI); (-)-(1R,2S)-(1,2-Epoxypropyl)phosphonicacid; (1R,2S)(-)-(1,2-Epoxypropyl)phosphonic acid disodium salt; (1R,2S)-(1,2-Epoxypropyl)phosphonic acid; (1R,2S)-epoxypropylphosphonate; (1R,2S)-epoxypropylphosphonic acid; (2R-cis)-(3-Methyloxiranyl)phosphonic acid disodium salt
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Bacterial infections [ICD9: 001-009, 010-018, 020-027, 030-041, 080-088, 090-099, 100-104; ICD10:A00-B99] | Approved | [1] | ||
| Therapeutic Class |
Antibiotics
|
||||
| Structure |
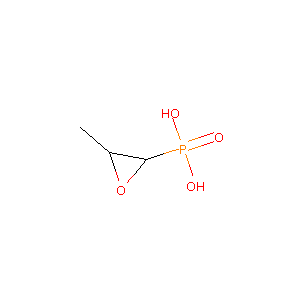
|
Download2D MOL |
|||
| Formula |
C3H7O4P
|
||||
| Canonical SMILES |
CC1C(O1)P(=O)(O)O
|
||||
| InChI |
1S/C3H7O4P/c1-2-3(7-2)8(4,5)6/h2-3H,1H3,(H2,4,5,6)/t2-,3+/m0/s1
|
||||
| InChIKey |
YMDXZJFXQJVXBF-STHAYSLISA-N
|
||||
| CAS Number |
CAS 23155-02-4
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
8687, 606108, 7887444, 8013956, 8027671, 10299988, 14747737, 15297304, 36889264, 46506665, 47206194, 50126493, 50767051, 57404778, 92298260, 93166606, 96099807, 103707732, 103934869, 104637332, 116933583, 124349057, 124385179, 129815629, 134338052, 134994616, 137006369, 140608615, 142971088, 160658318, 160964170, 162008097, 162496683, 164187510, 164187514, 164187516, 164187519, 164187529, 174551524, 175266349, 179150196, 226434285, 241044746, 250133924
|
||||
| SuperDrug ATC ID |
J01XX01
|
||||
| SuperDrug CAS ID |
cas=023155024
|
||||
| Drug Resistance Mutation (DRM) | |||||
| DRM | DRM Info | ||||
| Target and Pathway | |||||
| Target(s) | UDP-N-acetylglucosamine 1-carboxyvinyltransferase | Target Info | Inhibitor | [2], [3], [4], [5] | |
| References | |||||
| REF 1 | Has nature already identified all useful antibacterial targets? Curr Opin Microbiol. 2008 Oct;11(5):387-92. Epub 2008 Oct 6. | ||||
| REF 2 | Lysine 22 in UDP-N-acetylglucosamine enolpyruvyl transferase from Enterobacter cloacae is crucial for enzymatic activity and the formation of covalent adducts with the substrate phosphoenolpyruvate and the antibiotic fosfomycin. Biochemistry. 1999 Oct 5;38(40):13162-9. | ||||
| REF 3 | In vitro and in vivo functional activity of Chlamydia MurA, a UDP-N-acetylglucosamine enolpyruvyl transferase involved in peptidoglycan synthesis and fosfomycin resistance. J Bacteriol. 2003 Feb;185(4):1218-28. | ||||
| REF 4 | Conditional lethal amber mutations in essential Escherichia coli genes. J Bacteriol. 2004 May;186(9):2673-81. | ||||
| REF 5 | Evidence that the fosfomycin target Cys115 in UDP-N-acetylglucosamine enolpyruvyl transferase (MurA) is essential for product release. J Biol Chem. 2005 Feb 4;280(5):3757-63. Epub 2004 Nov 5. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

