Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D01JEU
|
||||
| Former ID |
DIB003515
|
||||
| Drug Name |
Memantine
|
||||
| Synonyms |
Memantine ER; Namenda XR; Memantine (extended release); Memantine (extended release), Forest
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Alzheimer disease [ICD9: 331; ICD10:G30] | Approved | [1], [2] | ||
| Company |
Merz & Co GmbH
|
||||
| Structure |
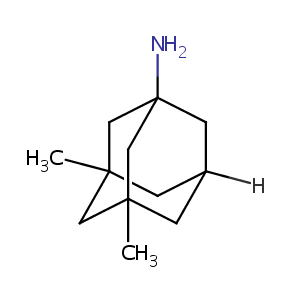
|
Download2D MOL |
|||
| Formula |
C12H21N
|
||||
| InChI |
InChI=1S/C12H21N/c1-10-3-9-4-11(2,6-10)8-12(13,5-9)7-10/h9H,3-8,13H2,1-2H3
|
||||
| InChIKey |
BUGYDGFZZOZRHP-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 19982-08-2
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
586325, 598200, 3221249, 6380039, 7737264, 8149719, 8152542, 11167042, 11335243, 11360482, 11363501, 11366063, 11368625, 11372025, 11374681, 11376787, 11461454, 11467006, 11468126, 11485192, 11486662, 11489224, 11490738, 11492836, 11494421, 15147301, 24310548, 26751885, 29223165, 46506702, 47290972, 47290973, 47440080, 47515155, 47662108, 47736297, 47736298, 48184834, 48259056, 48334316, 49698917, 50057972, 50104569, 53790630, 57322117, 74530305, 90341818, 96024864, 99301156, 103211988
|
||||
| Drug Resistance Mutation (DRM) | |||||
| DRM | DRM Info | ||||
| Target and Pathway | |||||
| Target(s) | NMDA receptor | Target Info | Modulator | [3], [4] | |
| References | |||||
| REF 1 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4253). | ||||
| REF 2 | Treatment effect size of memantine therapy in Alzheimer disease and vascular dementia. Alzheimer Dis Assoc Disord. 2006 Jul-Sep;20(3):133-7. | ||||
| REF 3 | The NMDA receptor antagonist memantine as a symptomatological and neuroprotective treatment for Alzheimer's disease: preclinical evidence. Int J Geriatr Psychiatry. 2003 Sep;18(Suppl 1):S23-32. | ||||
| REF 4 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

