Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D01KKQ
|
||||
| Former ID |
DAP000759
|
||||
| Drug Name |
Raltitrexed
|
||||
| Synonyms |
Tomudex; Arkomedika brand of raltitrexed; AstraZeneca brand of raltitrexed; Zeneca brand of raltitrexed; D 1694; ZD 1694; ZD1694; D-1694; KS-5069; SA-Detection Disk Kit; Tomudex (TN); ZD-16; ZD-1694; ZN-D1694; Raltitrexed (JAN/USAN/INN); Tomudex, TDX, ZD 1694, Raltitrexed; N-(5-(((3,4-Dihydro-2-methyl-4-oxo-6-quinazolinyl)methyl)methylamino)-2-thenoyl)-L-glutamic acid; N-(5-(N-(3,4-Dihydro-2-methyl-4-oxoquinazolin-6-ylmethyl)-N-methylamino)-2-thenoyl)-L-glutamic acid; (2S)-2-[[5-[methyl-[(2-methyl-4-oxo-1H-quinazolin-6-yl)methyl]amino]thiophene-2-carbonyl]amino]pentanedioic acid; (S)-2-[(1-{5-[Methyl-(2-methyl-4-oxo-3,4-dihydro-quinazolin-6-ylmethyl)-amino]-thiophen-2-yl}-methanoyl)-amino]-pentanedioic acid
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Anticancer Agents
|
||||
| Company |
AstraZeneca
|
||||
| Structure |
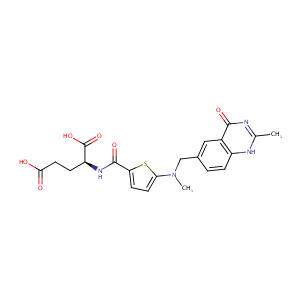
|
Download2D MOL |
|||
| Formula |
C21H22N4O6S
|
||||
| InChI |
InChI=1S/C21H22N4O6S/c1-11-22-14-4-3-12(9-13(14)19(28)23-11)10-25(2)17-7-6-16(32-17)20(29)24-15(21(30)31)5-8-18(26)27/h3-4,6-7,9,15H,5,8,10H2,1-2H3,(H,24,29)(H,26,27)(H,30,31)(H,22,23,28)/t15-/m0/s1
|
||||
| InChIKey |
IVTVGDXNLFLDRM-HNNXBMFYSA-N
|
||||
| CAS Number |
CAS 112887-68-0
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
13546, 499545, 643863, 822152, 832006, 834766, 7848127, 7886856, 10233592, 11417383, 12014189, 14906899, 16528454, 16861576, 44434371, 46386823, 46392177, 46394312, 46394338, 46504880, 46518849, 49681782, 49972881, 50445232, 53788217, 57337824, 81092886, 87568168, 91615849, 92308617, 92717646, 96100342, 99436931, 103512884, 104372860, 118855342, 124659060, 124757069, 124939249, 125163873, 126592925, 126670692, 127341460, 127341461, 128227540, 131480725, 134338044, 135060746, 135659878, 136342515
|
||||
| SuperDrug ATC ID |
L01BA03
|
||||
| SuperDrug CAS ID |
cas=112887680
|
||||
| Target and Pathway | |||||
| Target(s) | Thymidylate synthase | Target Info | Inhibitor | [534940], [536842] | |
| BioCyc Pathway | Pyrimidine deoxyribonucleotides biosynthesis from CTP | ||||
| Pyrimidine deoxyribonucleotides de novo biosynthesis | |||||
| Superpathway of pyrimidine deoxyribonucleotides de novo biosynthesis | |||||
| Superpathway of pyrimidine deoxyribonucleoside salvage | |||||
| DTMP de novo biosynthesis (mitochondrial) | |||||
| Pyrimidine deoxyribonucleosides salvage | |||||
| Pathway Interaction Database | E2F transcription factor network | ||||
| PathWhiz Pathway | Pyrimidine Metabolism | ||||
| WikiPathways | Trans-sulfuration and one carbon metabolism | ||||
| Retinoblastoma (RB) in Cancer | |||||
| One Carbon Metabolism | |||||
| Integrated Pancreatic Cancer Pathway | |||||
| miR-targeted genes in muscle cell - TarBase | |||||
| miR-targeted genes in lymphocytes - TarBase | |||||
| miR-targeted genes in leukocytes - TarBase | |||||
| miR-targeted genes in epithelium - TarBase | |||||
| Metabolism of nucleotides | |||||
| Fluoropyrimidine Activity | |||||
| References | |||||
| Ref 536361 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. Epub 2007 Feb 20. | ||||
| Ref 542426 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7403). | ||||
| Ref 534940 | Cellular pharmacology of MTA: a correlation of MTA-induced cellular toxicity and in vitro enzyme inhibition with its effect on intracellular folate and nucleoside triphosphate pools in CCRF-CEM cells. Semin Oncol. 1999 Apr;26(2 Suppl 6):48-54. | ||||
| Ref 536842 | DNA damage and homologous recombination signaling induced by thymidylate deprivation. Biochem Pharmacol. 2008 Oct 15;76(8):987-96. Epub 2008 Aug 19. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

