Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D01QIN
|
||||
| Former ID |
DAP000553
|
||||
| Drug Name |
Atorvastatin
|
||||
| Synonyms |
Atogal; Atorlip; Atorpic; Atorvastatina; Atorvastatine; Atorvastatinium; Atrovastin; Cardyl; Faboxim; Lipotropic; Lipovastatinklonal; Liprimar; Lowden; Sincol; Sortis; Sotis; Torvacard; Torvast; Totalip; Tozalip; Tulip; Vastina; Xanator; Xarator; Xavator; Zurinel; Atorvastatin (INN); Atorvastatin [INN:BAN]; Lipitor (TN); Lipitor(TM); Sortis (TN); (3R,5R)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-propan-2-ylpyrrol-1-yl]-3,5-dihydroxyheptanoic acid; (3R,5R)-7-[3-(anilinocarbonyl)-5-(4-fluorophenyl)-2-isopropyl-4-phenyl-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoic acid; (R-(R*,R*))-2-(4-Fluorophenyl)-beta,delta-dihydroxy-5-(1-methylethyl)-3-phenyl-4-((phenylamino)carbonyl)-1H-pyrrole-1-heptanoic acid; (betaR,deltaR)-2-(p-Fluorophenyl)-beta,delta-dihydroxy-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrole-1-heptanoic acid; 7-[2-(4-FLUORO-PHENYL)-5-ISOPROPYL-3-PHENYL-4-PHENYLCARBAMOYL-PYRROL-1-YL]-3,5-DIHYDROXY-HEPTANOIC ACID
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Cardiovascular disease; Hyperlipidemia; Dyslipidemia [ICD10:I00-I99, E78] | Approved | [551871] | ||
| Therapeutic Class |
Anticholesteremic Agents
|
||||
| Company |
Pfizer Pharmaceuticals
|
||||
| Structure |
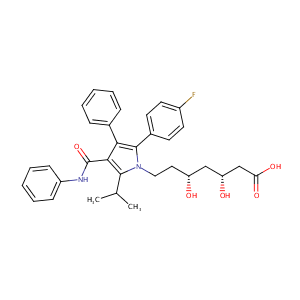
|
Download2D MOL |
|||
| Formula |
C33H35FN2O5
|
||||
| InChI |
InChI=1S/C33H35FN2O5/c1-21(2)31-30(33(41)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-26(37)19-27(38)20-28(39)40/h3-16,21,26-27,37-38H,17-20H2,1-2H3,(H,35,41)(H,39,40)/t26-,27-/m1/s1
|
||||
| InChIKey |
XUKUURHRXDUEBC-KAYWLYCHSA-N
|
||||
| CAS Number |
CAS 134523-00-5
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9052, 822166, 7884979, 7978735, 8187078, 14910832, 26684326, 26697359, 29215408, 43118161, 49845979, 50037926, 51091801, 53787715, 56311281, 56311722, 56311943, 56311969, 56312802, 56313042, 56313334, 56313578, 56314463, 57314133, 75432172, 85856289, 92714388, 93166494, 103554720, 104178840, 104321734, 104829550, 117367061, 124892211, 126525305, 126624171, 126658151, 126682126, 127315782, 127315783, 127315784, 127315785, 127315786, 127315787, 127315788, 127315789, 127315790, 127315791, 127315792, 127315793
|
||||
| ChEBI ID |
ChEBI:39548
|
||||
| SuperDrug ATC ID |
C10AA05
|
||||
| SuperDrug CAS ID |
cas=134523005
|
||||
| Target and Pathway | |||||
| Target(s) | 3-hydroxy-3-methylglutaryl-coenzyme A reductase | Target Info | Inhibitor | [535246] | |
| PANTHER Pathway | Cholesterol biosynthesis | ||||
| PathWhiz Pathway | Steroid Biosynthesis | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

