Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D01XNB
|
||||
| Former ID |
DAP000180
|
||||
| Drug Name |
Omeprazole
|
||||
| Synonyms |
AULCER; Antra; Audazol; Axagon; Belmazol; Ceprandal; Danlox; Demeprazol; Desec; Dizprazol; Dudencer; Elgam; Emeproton; Emilok; Epirazole; Erbolin; Esomeprazol; Esomeprazole; Esomperazole; Esopral; Exter; Gasec; Gastrimut; Gastroloc; Gibancer; Indurgan; Inhibitron; Inhipump; Lensor; Logastric; Lomac; Losec; Lucen; Mepral; Miol; Miracid; Mopral; Morecon; Nexium; Nilsec; Nopramin; Nuclosina; OMEP; OMZ; Ocid; Olexin; Olit; Omapren; Omebeta; Omed; Omegast; Omepradex; Omepral; Omeprazol; Omeprazolum; Omeprazon; Omeprazone; Omeprol; Omesek; Omez; Omezol; Omezolan; Omid; Omisec; Omizac; Ompanyt; Ortanol; Osiren; Ozoken; Paprazol; Parizac; Pepticum; Pepticus; Peptilcer; Prazentol; Prazidec; Prazolit; Prilosec; Procelac; Proclor; Prysma; Ramezol; Regulacid; Result; Sanamidol; Secrepina; Ulceral; Ulcesep; Ulcometion; Ulcozol; Ulcsep; Ulsen; Ultop; Ulzol; Victrix; Zefxon; Zegerid; Zepral; Zimor; Zoltum; Antra MUPS; Nexium IV; Omeprazole magnesium; Prilosec OTC; Tedec Ulceral; O0359; Omebeta 20; AGI-010; DM-3458; H 168-68; H 168/68; Losec (TN); Omeprazol [INN-Spanish]; Omeprazole delayed-release; Omeprazolum [INN-Latin]; Prilosec (TN); SAN-15; H-168/68; Omeprazole (JAN/USP/INN); Omeprazole [USAN:INN:BAN:JAN]; Losec, Omesec, Prilosec, Zegerid, Omeprazole; (-)-Omeprazole; 2-(((3,5-Dimethyl-4-methoxy-2-pyridyl)methyl)sulfinyl)-5-methoxy-1H-benzimidazole; 2-({[3,5-dimethyl-4-(methyloxy)pyridin-2-yl]methyl}sulfinyl)-5-(methyloxy)-1H-benzimidazole; 5-Methoxy-2-(((4-methoxy-3,5-dimethyl-2-pyridyl)methyl)sulfinyl)benzimidazole; 5-Methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole; 5-Methoxy-2[(4-methoxy-3,5-dimethyl-2-pyridyl)methylsulfinyl]-1H-benzimidazole; 5-methoxy-2-{[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfinyl}-1H-benzimidazole; 6-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methylsulfinyl]-1H-benzimidazole
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Antiulcer Agents
|
||||
| Company |
AstraZeneca
|
||||
| Structure |
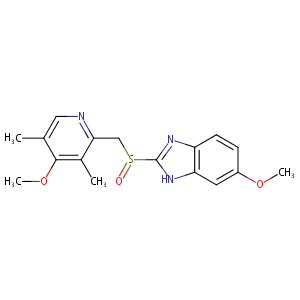
|
Download2D MOL |
|||
| Formula |
C17H19N3O3S
|
||||
| InChI |
InChI=1S/C17H19N3O3S/c1-10-8-18-15(11(2)16(10)23-4)9-24(21)17-19-13-6-5-12(22-3)7-14(13)20-17/h5-8H,9H2,1-4H3,(H,19,20)
|
||||
| InChIKey |
SUBDBMMJDZJVOS-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 73590-58-6
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9532, 640212, 855576, 3281991, 4696242, 5212304, 7847521, 7980199, 8152819, 10321754, 11112840, 11398365, 11466521, 11467641, 11486042, 11528599, 12013167, 14753960, 14875946, 22391484, 24897870, 26751452, 26751453, 26759296, 29223683, 46386973, 46487906, 46509065, 47365309, 47589088, 47589089, 48035237, 48170542, 48414255, 48416351, 49681572, 49698521, 49834935, 50100529, 50103932, 53788700, 56313300, 56313696, 56313928, 56422106, 57322340, 80295314, 81093370, 85174452, 85788870
|
||||
| ChEBI ID |
ChEBI:7772
|
||||
| SuperDrug ATC ID |
A02BC01
|
||||
| SuperDrug CAS ID |
cas=073590586
|
||||
| Target and Pathway | |||||
| Target(s) | H+ K+ ATPase | Target Info | Modulator | [556264] | |
| References | |||||
| Ref 467616 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4279). | ||||
| Ref 537527 | Generic omeprazole delayed-release capsules: in vitro performance evaluations. Drug Dev Ind Pharm. 2009 Aug;35(8):917-21. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

