Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D01ZII
|
||||
| Former ID |
DAP000374
|
||||
| Drug Name |
Chlorpromazine
|
||||
| Synonyms |
Aminasine; Aminazin; Aminazine; Ampliactil; Amplicitil; Amplictil; Chlordelazine; Chlorderazin; Chloropromazine; Chlorpromados; Chlorpromazin; Chlorpromazinum; Clorpromazina; Contomin; Cromedazine; Elmarin; Esmind; Fenactil; Fenaktyl; Largactil; Largactilothiazine; Largactyl; Megaphen; Novomazina; Phenactyl; Phenathyl; Plegomasine; Plegomazin; Prazilpromactil; Proma; Promactil; Promazil; Propaphen; Propaphenin; Prozil; Psychozine; Sanopron; Thorazine; Torazina; Wintermin; Chlorpromazine Tannate; Clorpromazina [Italian]; Fraction AB; Largactil Liquid; Largactil Oral Drops; Phenothiazine hydrochloride; Thorazine Spansule; Thorazine Suppositories; Thorazine hydrochloride; BC 135; HL 5746; JHICC02042; SKF 2601A; Z80; Chlor-PZ; Chlor-Promanyl; Chlorpromanyl (discontinued); Chlorpromazinum [INN-Latin]; Clorpromazina [INN-Spanish]; Largactil (TN); Novo-Chlorpromazine; SKF 2601-A; SKF-2601; Thorazine (TN); Chlorpromazine (USP/INN); Chlorpromazine [USAN:INN:BAN]; Chloro-3 (dimethylamino-3 propyl)-10 phenothiazine; Chloro-3 (dimethylamino-3 propyl)-10 phenothiazine [French]; N-(3-Dimethylaminopropyl)-3-chlorophenothiazine; [3-(2-Chloro-phenothiazin-10-yl)-propyl]-dimethyl-amine; 10-(3-Dimethylaminopropyl)-2-chlorophenothiazine; 10H-Phenothiazine-10-propanamine, 2-chloro-N,N-dimethyl-, radical ion(1+); 2-Chloro-10-(3-(dimethylamino)propyl)phenothiazine; 2-Chloro-10-[3-(dimethylamino)propyl]phenothiazine; 2-Chloropromazine; 2-Cloro-10 (3-dimetilaminopropil)fenotiazina; 2-Cloro-10 (3-dimetilaminopropil)fenotiazina [Italian]; 2-chloro-10-(3-(dimethylamino)propyl)-phenothiazine; 2-chloro-N,N-dimethyl-10H-Phenothiazine-10-propanamine; 3-(2-Chloro-10H-phenothiazin-10-yl)-N,N-dimethyl-1-propanamine; 3-(2-chloro-10H-phenothiazin-10-yl)-N,N-dimethylpropan-1-amine; 3-(2-chlorophenothiazin-10-yl)-N,N-dimethyl-propan-1-amine; 3-(2-chlorophenothiazin-10-yl)-N,N-dimethylpropan-1-amine
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Antipsychotic Agents
|
||||
| Company |
GlaxoSmithKline
|
||||
| Structure |
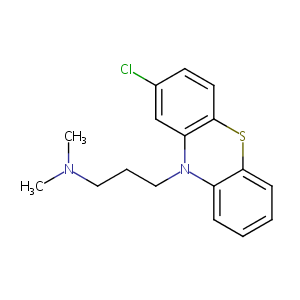
|
Download2D MOL |
|||
| Formula |
C17H19ClN2S
|
||||
| InChI |
InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3
|
||||
| InChIKey |
ZPEIMTDSQAKGNT-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 50-53-3
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9123, 441206, 602724, 841999, 4332427, 7847336, 7978926, 8149253, 8151764, 10524433, 10588932, 11110990, 11110991, 11120337, 11120825, 11121313, 11335799, 11361038, 11363036, 11364725, 11365598, 11367287, 11368160, 11369849, 11371331, 11372890, 11373945, 11375449, 11376322, 11378013, 11462010, 11466092, 11467212, 11484847, 11485904, 11488972, 11490162, 11492098, 11493956, 12014508, 14923746, 24398080, 25688547, 26611657, 26680205, 26747094, 26747095, 26751620, 26751621, 29221882
|
||||
| ChEBI ID |
ChEBI:3647
|
||||
| SuperDrug ATC ID |
N05AA01
|
||||
| SuperDrug CAS ID |
cas=000050533
|
||||
| Target and Pathway | |||||
| Target(s) | D(2) dopamine receptor | Target Info | Antagonist | [537110], [537239], [538131] | |
| References | |||||
| Ref 538339 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 080439. | ||||
| Ref 543051 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 83). | ||||
| Ref 537110 | Basolateral amygdala D1- and D2-dopaminergic system promotes the formation of long-term potentiation in the dentate gyrus of anesthetized rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009 Apr 30;33(3):552-6. Epub 2009 Feb 23. | ||||
| Ref 537239 | Modulatory role of dopamine D2 receptors and fundamental role of L-type Ca2+ channels in the induction of long-term potentiation in the basolateral amygdala-dentate gyrus pathway of anesthetized rats. Eur J Pharmacol. 2009 Mar 15;606(1-3):90-3. Epub 2009 Jan 22. | ||||
| Ref 538131 | Behavioral and neurochemical methods in research on new psychotropics. Ann Pharm Fr. 1998;56(2):54-9. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

