Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D02GOH
|
||||
| Former ID |
DNC006015
|
||||
| Drug Name |
AM-404
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Discovery agent | Investigative | [534262] | ||
| Structure |
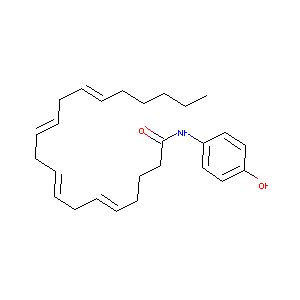
|
Download2D MOL |
|||
| Formula |
C26H37NO2
|
||||
| Canonical SMILES |
CCCCCC=CCC=CCC=CCC=CCCCC(=O)NC1=CC=C(C=C1)O
|
||||
| InChI |
1S/C26H37NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-26(29)27-24-20-22-25(28)23-21-24/h6-7,9-10,12-13,15-16,20-23,28H,2-5,8,11,14,17-19H2,1H3,(H,27,29)/b7-6+,10-9+,13-12+,16-15+
|
||||
| InChIKey |
IJBZOOZRAXHERC-CGRWFSSPSA-N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Cannabinoid receptor 2 | Target Info | Inhibitor | [529838] | |
| Cannabinoid receptor 1 | Target Info | Inhibitor | [534262] | ||
| Fatty-acid amide hydrolase | Target Info | Inhibitor | [529838] | ||
| BioCyc Pathway | Anandamide degradation | ||||
| PANTHER Pathway | Endogenous cannabinoid signalingP05728:Anandamide degradation | ||||
| Pathway Interaction Database | N-cadherin signaling events | ||||
| References | |||||
| Ref 529838 | J Med Chem. 2008 Dec 25;51(24):7800-5.New analgesics synthetically derived from the paracetamol metabolite N-(4-hydroxyphenyl)-(5Z,8Z,11Z,14Z)-icosatetra-5,8,11,14-enamide. | ||||
| Ref 534262 | J Med Chem. 1996 Oct 25;39(22):4515-9.Head group analogs of arachidonylethanolamide, the endogenous cannabinoid ligand. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

