Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D02GWF
|
||||
| Former ID |
DNC009514
|
||||
| Drug Name |
PACTIMIBE
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Arteriosclerosis [ICD9: 440; ICD10:I70] | Phase 2/3 | [1] | ||
| Structure |
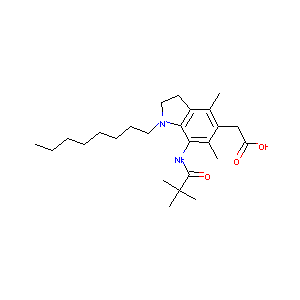
|
Download2D MOL |
|||
| Formula |
C25H40N2O3
|
||||
| Canonical SMILES |
CCCCCCCCN1CCC2=C(C(=C(C(=C21)NC(=O)C(C)(C)C)C)CC(=O)O)C
|
||||
| InChI |
1S/C25H40N2O3/c1-7-8-9-10-11-12-14-27-15-13-19-17(2)20(16-21(28)29)18(3)22(23(19)27)26-24(30)25(4,5)6/h7-16H2,1-6H3,(H,26,30)(H,28,29)
|
||||
| InChIKey |
TXIIZHHIOHVWJD-UHFFFAOYSA-N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Liver carboxylesterase | Target Info | Inhibitor | [2] | |
| KEGG Pathway | Drug metabolism - other enzymes | ||||
| Metabolic pathways | |||||
| Pathway Interaction Database | E2F transcription factor network | ||||
| WikiPathways | NRF2 pathway | ||||
| Nuclear Receptors Meta-Pathway | |||||
| Heroin metabolism | |||||
| Irinotecan Pathway | |||||
| Fluoropyrimidine Activity | |||||
| Phase I biotransformations, non P450 | |||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT00151788) Efficacy and Safety of the ACAT Inhibitor CS-505 (Pactimibe) for Reducing the Progression of Carotid Artery Disease. This Study is Also Known as CAPTIVATE.. U.S. National Institutes of Health. | ||||
| REF 2 | J Med Chem. 2008 Aug 14;51(15):4823-33. Epub 2008 Jul 12.Novel indoline-based acyl-CoA:cholesterol acyltransferase inhibitor with antiperoxidative activity: improvement of physicochemical propertiesand biological activities by introduction of carboxylic acid. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

