Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D02HBB
|
||||
| Former ID |
DNCL002590
|
||||
| Drug Name |
AEZS-108
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Acute lymphoblastic leukemia [ICD9: 204.0, 556; ICD10:C91.0] | Phase 3 | [524186] | ||
| Company |
AEterna Zentaris
|
||||
| Structure |
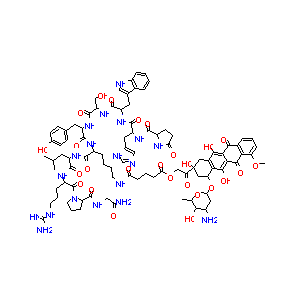
|
Download2D MOL |
|||
| Formula |
C91H117N19O26
|
||||
| Canonical SMILES |
CC1C(C(CC(O1)OC2CC(CC3=C(C4=C(C(=C23)O)C(=O)C5=C(C4=O)C<br />=CC=C5OC)O)(C(=O)COC(=O)CCCC(=O)NCCCCC(C(=O)NC(CC(C)C)C<br />(=O)NC(CCCNC(=N)N)C(=O)N6CCCC6C(=O)NCC(=O)N)NC(=O)C(CC7<br />=CC=C(C=C7)O)NC(=O)C(CO)NC(=O)C(CC8=CNC9=CC=CC=C98)NC(=<br />O)C(CC1=CN=CN1)NC(=O)C1CCC(=O)N1)O)N)O
|
||||
| InChI |
1S/C91H117N19O26/c1-44(2)31-58(83(125)104-57(17-11-29-98-90(94)95)89(131)110-30-12-18-63(110)88(130)100-40-67(93)114)105-81(123)55(16-7-8-28-97-68(115)20-10-21-70(117)134-42-66(113)91(132)36-52-73(65(37-91)136-71-35-53(92)76(118)45(3)135-71)80(122)75-74(78(52)120)77(119)51-14-9-19-64(133-4)72(51)79(75)121)103-84(126)59(32-46-22-24-49(112)25-23-46)106-87(129)62(41-111)109-85(127)60(33-47-38-99-54-15-6-5-13-50(47)54)107-86(128)61(34-48-39-96-43-101-48)108-82(124)56-26-27-69(116)102-56/h5-6,9,13-15,19,22-25,38-39,43-45,53,55-63,65,71,76,99,111-112,118,120,122,132H,7-8,10-12,16-18,20-21,26-37,40-42,92H2,1-4H3,(H2,93,114)(H,96,101)(H,97,115)(H,100,130)(H,102,116)(H,103,126)(H,104,125)(H,105,123)(H,106,129)(H,107,128)(H,108,124)(H,109,127)(H4,94,95,98)/t45-,53-,55+,56-,57-,58-,59-,60-,61-,62-,63-,65-,71-,76+,91-/m0/s1
|
||||
| InChIKey |
OOUACICUAVTCEC-LZHWUUGESA-N
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| Target and Pathway | |||||
| Target(s) | Gonadotropin-releasing hormone receptor | Target Info | Modulator | [525681] | |
| NetPath Pathway | IL1 Signaling Pathway | ||||
| IL2 Signaling Pathway | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

