Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D02HUB
|
||||
| Former ID |
DIB011230
|
||||
| Drug Name |
Nestorone transdermal spray
|
||||
| Synonyms |
Nestorone; Nestorone MDTS; Nestorone metered dose transdermal system; Nestorone transdermal spray, Acrux; ST-1435; Nestorone transdermal spray, Population Council/Acrux
|
||||
| Indication | Endometriosis [ICD9: 617; ICD10:N80] | Phase 3 | [1] | ||
| Company |
Population Council; Population Council Center for Biomedical Research
|
||||
| Structure |
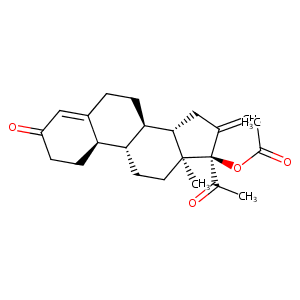
|
Download2D MOL |
|||
| Canonical SMILES |
[C@]12([C@@](C(=C)C[C@H]1[C@H]1[C@@H]([C@@H]3C(=CC(=O)C<br />C3)CC1)CC2)(OC(=O)C)C(=O)C)C
|
||||
| CAS Number |
CAS 7759-35-5
|
||||
| Target and Pathway | |||||
| Target(s) | Progesterone receptor | Target Info | Agonist | [2] | |
| KEGG Pathway | Oocyte meiosis | ||||
| Progesterone-mediated oocyte maturation | |||||
| Pathway Interaction Database | Cellular roles of Anthrax toxin | ||||
| Reactome | Nuclear signaling by ERBB4 | ||||
| Nuclear Receptor transcription pathway | |||||
| WikiPathways | Ovarian Infertility Genes | ||||
| Signaling by ERBB4 | |||||
| Nuclear Receptors | |||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT00455156) Study of the Safety, Efficacy and Cycle Control of a Contraceptive Vaginal Ring. U.S. National Institutes of Health. | ||||
| REF 2 | Efficacy of the selective progesterone receptor agonist Nestorone for chronic experimental autoimmune encephalomyelitis. J Neuroimmunol. 2014 Nov 15;276(1-2):89-97. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

