Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D02OTK
|
||||
| Former ID |
DNCL001654
|
||||
| Drug Name |
CH-4051
|
||||
| Indication | Rheumatoid arthritis [ICD9: 710-719, 714; ICD10:M05-M06] | Phase 2 | [523026] | ||
| Company |
Chelsea Therapeutics
|
||||
| Structure |
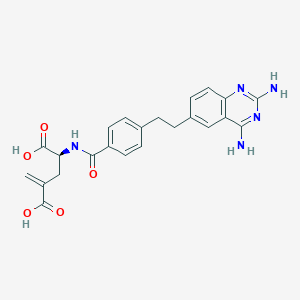
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | DHFR | Target Info | Inhibitor | [531111] | |
| PANTHER Pathway | Tetrahydrofolate biosynthesis | ||||
| Formyltetrahydroformate biosynthesis | |||||
| Pathway Interaction Database | E2F transcription factor network | ||||
| PathWhiz Pathway | Folate Metabolism | ||||
| Pterine Biosynthesis | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

