Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D02UKX
|
||||
| Former ID |
DIB015315
|
||||
| Drug Name |
CAP1-6D
|
||||
| Synonyms |
Modified CEA peptide (pancreatic cancer), University of Chicago
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Pancreatic cancer [ICD9: 140-199, 140-229, 157, 210-229; ICD10:C25] | Phase 2 | [521707] | ||
| Company |
University of Chicago
|
||||
| Structure |
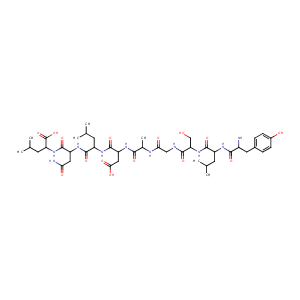
|
Download2D MOL |
|||
| Formula |
C43H68N10O15
|
||||
| Canonical SMILES |
CC(C)CC(C(=O)NC(CO)C(=O)NCC(=O)NC(C)C(=O)NC(CC(=O)O)C(=<br />O)NC(CC(C)C)C(=O)NC(CC(=O)N)C(=O)NC(CC(C)C)C(=O)O)NC(=O<br />)C(CC1=CC=C(C=C1)O)N
|
||||
| InChI |
1S/C43H68N10O15/c1-20(2)12-27(49-37(61)26(44)15-24-8-10-25(55)11-9-24)40(64)53-32(19-54)38(62)46-18-34(57)47-23(7)36(60)48-30(17-35(58)59)42(66)50-28(13-21(3)4)39(63)51-29(16-33(45)56)41(65)52-31(43(67)68)14-22(5)6/h8-11,20-23,26-32,54-55H,12-19,44H2,1-7H3,(H2,45,56)(H,46,62)(H,47,57)(H,48,60)(H,49,61)(H,50,66)(H,51,63)(H,52,65)(H,53,64)(H,58,59)(H,67,68)
|
||||
| InChIKey |
SNKUSVNHTCUELQ-UHFFFAOYSA-N
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| Target and Pathway | |||||
| Target(s) | Carcinoembryonic antigen-related cell adhesion molecule 5 | Target Info | Modulator | [530852], [534511] | |
| NetPath Pathway | TGF_beta_Receptor Signaling Pathway | ||||
| References | |||||
| Ref 530852 | HLA-A*0201-restricted CEA-derived peptide CAP1 is not a suitable target for T-cell-based immunotherapy. J Immunother. 2010 May;33(4):402-13. | ||||
| Ref 534511 | Identification of an enhancer agonist cytotoxic T lymphocyte peptide from human carcinoembryonic antigen. Cancer Res. 1997 Oct 15;57(20):4570-7. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

