Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D02XXN
|
||||
| Former ID |
DNCL003802
|
||||
| Drug Name |
Evacetrapib
|
||||
| Synonyms |
LY2484595
|
||||
| Drug Type |
Small molecular drug
|
||||
| Company |
Lilly
|
||||
| Structure |
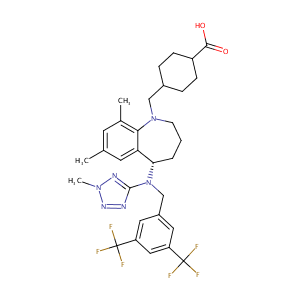
|
Download2D MOL |
|||
| Formula |
C31H36F6N6O2
|
||||
| InChI |
InChI=1S/C31H36F6N6O2/c1-18-11-19(2)27-25(12-18)26(5-4-10-42(27)16-20-6-8-22(9-7-20)28(44)45)43(29-38-40-41(3)39-29)17-21-13-23(30(32,33)34)15-24(14-21)31(35,36)37/h11-15,20,22,26H,4-10,16-17H2,1-3H3,(H,44,45)/t20?,22?,26-/m0/s1
|
||||
| InChIKey |
IHIUGIVXARLYHP-UXNJHFGPSA-N
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
103911733, 135268436, 135626839, 137240261, 141386796, 141386797, 152258542, 160647376, 160699241, 160860498, 161005253, 162221695, 164194109, 170504191, 172096382, 198940733, 223390433, 223471431, 224462115, 225848218, 226482340, 226482535, 226508337, 244317218, 248331392, 249734743, 252155357, 252160892, 252166611, 252215734, 252471646, 252810963
|
||||
| Target and Pathway | |||||
| Target(s) | Cholesteryl ester transfer protein | Target Info | Inhibitor | [532996] | |
| PANTHER Pathway | CCKR signaling map ST | ||||
| References | |||||
| Ref 524894 | ClinicalTrials.gov (NCT02227784) A Study of Evacetrapib (LY2484595) in Participants With High Cholesterol. U.S. National Institutes of Health. | ||||
| Ref 543108 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8401). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

