Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D03KOZ
|
||||
| Former ID |
DAP001376
|
||||
| Drug Name |
Epalrestat
|
||||
| Synonyms |
Epalrestatum; Kinedak; Epalrestat [INN]; Epalrestatum [Latin]; ONO 2; Ono 2235; Aldorin (TN); Kinedak (TN); ONO-2; Ono-2235; Epalrestat (JAN/INN); {5-[(E)-2-Methyl-3-phenyl-prop-2-en-(Z)-ylidene]-4-oxo-2-thioxo-thiazolidin-3-yl}-acetic acid; {(5Z)-5-[(2E)-2-methyl-3-phenylprop-2-en-1-ylidene]-4-oxo-2-thioxo-1,3-thiazolidin-3-yl}acetic acid; 2-((z)-5-((e)-2-methyl-3-phenylallylidene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid; 2-[(5Z)-5-[(E)-2-methyl-3-phenylprop-2-enylidene]-4-oxo-2-sulfanylidene-1,3-thiazolidin-3-yl]acetic acid; 3-carboxymethyl-5-(methyl-3-phenylpropenylidene)rhodanine; 5-((1Z,2E)-2-Methyl-3-phenylpropenylidene)-4-oxo-2-thioxo-3-thiazolidineacetic acid; 5-((Z,E)-beta-Methylcinnamylidene)-4-oxo-2-thioxo-3-thiazolidineacetic acid
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Diabetic neuropathy [ICD9: 250, 250.6, 356.0, 356.8; ICD10:E11.40] | Approved | [536361] | ||
| Therapeutic Class |
Hypoglycemic Agents
|
||||
| Company |
Eskayef Bangladesh Limited
|
||||
| Structure |
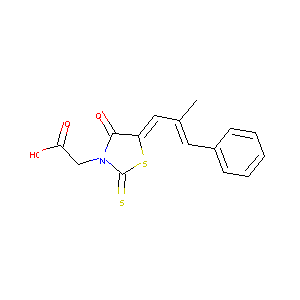
|
Download2D MOL |
|||
| Formula |
C15H13NO3S2
|
||||
| Canonical SMILES |
CC(=CC1=CC=CC=C1)C=C2C(=O)N(C(=S)S2)CC(=O)O
|
||||
| InChI |
1S/C15H13NO3S2/c1-10(7-11-5-3-2-4-6-11)8-12-14(19)16(9-13(17)18)15(20)21-12/h2-8H,9H2,1H3,(H,17,18)/b10-7+,12-8-
|
||||
| InChIKey |
CHNUOJQWGUIOLD-NFZZJPOKSA-N
|
||||
| CAS Number |
CAS 82159-09-9
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
7848751, 8653343, 12012832, 14826092, 26664249, 26758035, 32249007, 50052031, 57409089, 57974228, 79306115, 81092897, 85171753, 87932284, 103245600, 103980041, 104822630, 110525354, 117664431, 125126796, 126684126, 130673647, 134223203, 134338587, 135026827, 135692379, 136374773, 136946437, 137249280, 137267392, 142773098, 143404361, 143970327, 144115945, 162035741, 162177691, 164196566, 172089065, 172120909, 174478174, 175267046, 179151242, 184015922, 184545249, 184816928, 188628128, 210276582, 210281429, 223381370, 223434916
|
||||
| Target and Pathway | |||||
| Target(s) | Aldose reductase | Target Info | Inhibitor | [535244], [537989] | |
| NetPath Pathway | IL1 Signaling Pathway | ||||
| TGF_beta_Receptor Signaling Pathway | |||||
| References | |||||
| Ref 535244 | Long-term effect of epalrestat, an aldose reductase inhibitor, on the development of incipient diabetic nephropathy in Type 2 diabetic patients. J Diabetes Complications. 2001 Sep-Oct;15(5):241-4. | ||||
| Ref 537989 | Clinical investigation of epalrestat, an aldose reductase inhibitor, on diabetic neuropathy in Japan: multicenter study. Diabetic Neuropathy Study Group in Japan. J Diabetes Complications. 1996 May-Jun;10(3):168-72. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

