Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D03OGQ
|
||||
| Former ID |
DIB013151
|
||||
| Drug Name |
AZD-7009
|
||||
| Synonyms |
AR-H065522XX; AZD-7009; AZD-7009 (iv formulation, atrial fibrillation conversion)
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Atrial fibrillation [ICD9: 272, 427.31; ICD10:E78, I48] | Phase 2 | [521760] | ||
| Company |
AstraZeneca plc
|
||||
| Structure |
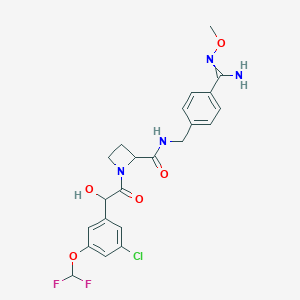
|
Download2D MOL |
|||
| Formula |
C23H34N4O5
|
||||
| Canonical SMILES |
N1(CC(=O)C(C)(C)C)CC2OC(C1)CN(C2)CCCNc1ccc(C#N)cc1
|
||||
| CAS Number |
CAS 335619-12-0
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Potassium channel | Target Info | Modulator | [532667] | |
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

