Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D04JPJ
|
||||
| Former ID |
DAP000991
|
||||
| Drug Name |
Lomustine
|
||||
| Synonyms |
Belustine; CCNU; CINU; Cecenu; CeeNU; Chloroethylcyclohexylnitrosourea; Lomustina; Lomustinum; Bristol Myers Squibb Brand of Lomustine; CCNU [Chloroethyl nitrosoureas]; Cyclohexyl chloroethyl nitrosourea; Lomustine medac Brand; Medac Brand of Lomustine; Rhone Poulenc Rorer Brand of Lomustine; OR5087; RB 1509; SRI 2200; Bristol-Myers Squibb Brand of Lomustine; CeeNU (TN); Lomustina [INN-Spanish]; Lomustinum [INN-Latin]; NPFAPI-06; Rhone-Poulenc Rorer Brand of Lomustine; CeeNU, CCNU, Lomustine; Lomustine (USAN/INN); Lomustine [USAN:BAN:INN]; N-(2-Chloroethyl)-N'-cyclohexyl-N-nitrosourea; (Chloro-2-ethyl)-1-cyclohexyl-3-nitrosourea; (Cloro-2-etil)-1-cicloesil-3-nitrosourea; (Cloro-2-etil)-1-cicloesil-3-nitrosourea [Italian];1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea; 1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea [Chloroethyl nitrosoureas]; 1-(2-Chloroethyl)-3-cyclohexylnitrosourea
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Brain cancer; Glioma [ICD9: 191, 225.0; ICD10:C71, D33] | Approved | [1], [2], [3] | ||
| Therapeutic Class |
Anticancer Agents
|
||||
| Structure |
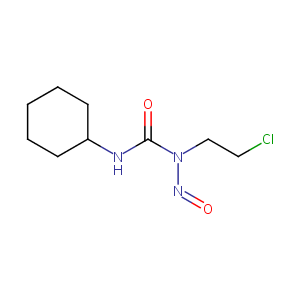
|
Download2D MOL |
|||
| Formula |
C9H16ClN3O2
|
||||
| InChI |
InChI=1S/C9H16ClN3O2/c10-6-7-13(12-15)9(14)11-8-4-2-1-3-5-8/h8H,1-7H2,(H,11,14)
|
||||
| InChIKey |
GQYIWUVLTXOXAJ-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 13010-47-4
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9290, 119147, 604677, 4964182, 7847429, 7979789, 8152480, 11446095, 11446868, 15121730, 29223064, 46506562, 48416181, 49854903, 50112720, 50177010, 53789456, 56313684, 57322064, 71821703, 81040920, 84941717, 92309013, 92710003, 103173774, 104253398, 104304994, 117664391, 118043410, 118049573, 124757517, 125164321, 125339834, 126533760, 126632297, 126658488, 126664336, 127993436, 131297250, 132048649, 134338261, 134989249, 135584134, 135692302, 136342506, 136375523, 136974470, 136999623, 137005631, 140170346
|
||||
| SuperDrug ATC ID |
L01AD02
|
||||
| SuperDrug CAS ID |
cas=013010474
|
||||
| Target and Pathway | |||||
| Target(s) | DNA | Target Info | Inhibitor | [4] | |
| References | |||||
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 017588. | ||||
| REF 2 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7214). | ||||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
| REF 4 | Synthesis and evaluation of ethylnitrosoureas of substituted naphthalimides as anticancer compounds. Acta Pol Pharm. 2007 Jan-Feb;64(1):27-33. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

