Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D05QHL
|
||||
| Former ID |
DCL000986
|
||||
| Drug Name |
Sivelestat sodium hydrate
|
||||
| Synonyms |
Elaspol; Sivelestat sodium; LY544349 Sodium Hydrate; Ono 5046; EI-546; Elaspol (TN); Sivelestat sodium (USAN); Sivelestat sodium hydrate (JAN); ONO-5046.Na; Sodium 2-[[2-[[4-(2,2-dimethylpropanoyloxy)phenyl]sulfonylamino]benzoyl]amino]acetate tetrahydrate; Sodium ((2-(((4-((2,2-dimethylpropanoyl)oxy)phenyl)sulfonyl)amino)benzoyl)amino)acetate tetrahydrate; Glycine, N-(2-(((4-(2,2-dimethyl-1-oxopropoxy)phenyl)sulfonyl)amino)benzoyl)-, monosodium salt, tetrahydrate
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Acute lung injury; Acute respiratory distress syndrome [ICD9: 518.5, 518.82; ICD10:J80] | Phase 4 | [536361] | ||
| Company |
ONO Pharmaceuticals
|
||||
| Structure |
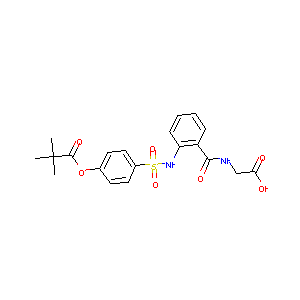
|
Download2D MOL |
|||
| Formula |
C20H29N2NaO11S
|
||||
| Canonical SMILES |
CC(C)(C)C(=O)OC1=CC=C(C=C1)S(=O)(=O)NC2=CC=CC=C2C(=O)NC<br />C(=O)[O-].O.O.O.O.[Na+]
|
||||
| InChI |
1S/C20H22N2O7S.Na.4H2O/c1-20(2,3)19(26)29-13-8-10-14(11-9-13)30(27,28)22-16-7-5-4-6-15(16)18(25)21-12-17(23)24;;;;;/h4-11,22H,12H2,1-3H3,(H,21,25)(H,23,24);;4*1H2/q;+1;;;;/p-1
|
||||
| InChIKey |
PLHREJBSQUSUCW-UHFFFAOYSA-M
|
||||
| CAS Number |
CAS 201677-61-4
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
7848980, 10253427, 12014432, 14934312, 46234170, 56039266, 57386948, 77060685, 92714191, 93302558, 113440824, 121278936, 126592378, 126624342, 126655598, 126670224, 134339418, 135114580, 144206123, 152040505, 152102776, 162181212, 165235284, 170465996, 184546383, 196106031, 198935522, 210275600, 210281259, 223394142, 223663430, 242585327, 252218912
|
||||
| Target and Pathway | |||||
| Target(s) | Leukocyte elastase | Target Info | Inhibitor | [536500] | |
| Pathway Interaction Database | Urokinase-type plasminogen activator (uPA) and uPAR-mediated signaling | ||||
| C-MYB transcription factor network | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

