Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D05TVT
|
||||
| Former ID |
DNC013001
|
||||
| Drug Name |
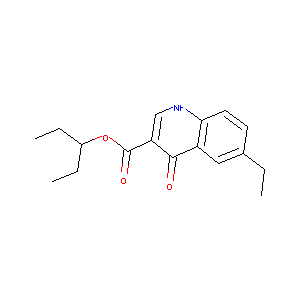
6-ethyl-3-(3-pentoxycarbonyl)-4-quinolone
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Discovery agent | Investigative | [1] | ||
| Structure |
|
Download2D MOL |
|||
| Formula |
C17H21NO3
|
||||
| Canonical SMILES |
CCC1=CC2=C(C=C1)NC=C(C2=O)C(=O)OC(CC)CC
|
||||
| InChI |
1S/C17H21NO3/c1-4-11-7-8-15-13(9-11)16(19)14(10-18-15)17(20)21-12(5-2)6-3/h7-10,12H,4-6H2,1-3H3,(H,18,19)
|
||||
| InChIKey |
OLWFMJLLBYGGKS-UHFFFAOYSA-N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Gamma-aminobutyric acid receptor | Target Info | Inhibitor | [1] | |
| Gamma-aminobutyric acid receptor subunit gamma-2 | Target Info | Inhibitor | [1] | ||
| Gamma-aminobutyric acid receptor subunit beta-2 | Target Info | Inhibitor | [1] | ||
| Gamma-aminobutyric acid receptor subunit alpha-1 | Target Info | Inhibitor | [1] | ||
| KEGG Pathway | Neuroactive ligand-receptor interaction | ||||
| Retrograde endocannabinoid signaling | |||||
| GABAergic synapse | |||||
| Morphine addiction | |||||
| Nicotine addictionhsa04080:Neuroactive ligand-receptor interaction | |||||
| Serotonergic synapse | |||||
| Nicotine addiction | |||||
| Reactome | Ligand-gated ion channel transport | ||||
| GABA A receptor activationR-HSA-975298:Ligand-gated ion channel transport | |||||
| GABA A receptor activation | |||||
| WikiPathways | Neurotransmitter Receptor Binding And Downstream Transmission In The Postsynaptic Cell | ||||
| Iron uptake and transportWP2754:Neurotransmitter Receptor Binding And Downstream Transmission In The Postsynaptic Cell | |||||
| Iron uptake and transportWP706:SIDS Susceptibility Pathways | |||||
| Iron uptake and transport | |||||
| References | |||||
| REF 1 | J Med Chem. 2006 Apr 20;49(8):2526-33.4-quinolone derivatives: high-affinity ligands at the benzodiazepine site of brain GABA A receptors. synthesis, pharmacology, and pharmacophore modeling. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

