Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D06HBT
|
||||
| Former ID |
DNCL001812
|
||||
| Drug Name |
LT-1951
|
||||
| Indication | Coronary artery disease [ICD9: 410-414, 429.2; ICD10:I20-I25] | Phase 1/2 | [521773] | ||
| Company |
Lumen Therapeutics
|
||||
| Structure |
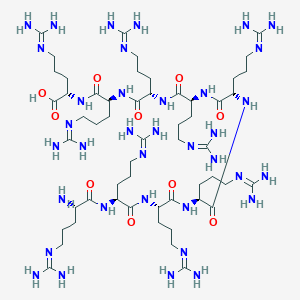
|
Download2D MOL |
|||
| Formula |
C54H110N36O10
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Nitric oxide synthase, inducible | Target Info | Modulator | [526624] | |
| BioCyc Pathway | Citrulline-nitric oxide cycle | ||||
| KEGG Pathway | Arginine biosynthesis | ||||
| Arginine and proline metabolism | |||||
| Metabolic pathways | |||||
| Calcium signaling pathway | |||||
| HIF-1 signaling pathway | |||||
| Peroxisome | |||||
| Salmonella infection | |||||
| Pertussis | |||||
| Leishmaniasis | |||||
| Chagas disease (American trypanosomiasis) | |||||
| Toxoplasmosis | |||||
| Amoebiasis | |||||
| Tuberculosis | |||||
| Pathways in cancer | |||||
| Small cell lung cancer | |||||
| NetPath Pathway | IL1 Signaling Pathway | ||||
| IL2 Signaling Pathway | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

