Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D06SME
|
||||
| Former ID |
DNC010929
|
||||
| Drug Name |
5-hydroxy-1-tosyl-1H-pyrrol-2(5H)-one
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Discovery agent | Investigative | [1] | ||
| Structure |
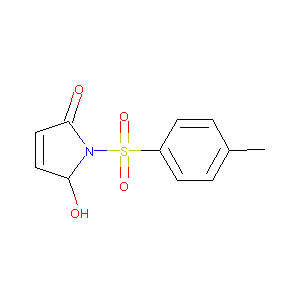
|
Download2D MOL |
|||
| Formula |
C11H11NO4S
|
||||
| Canonical SMILES |
CC1=CC=C(C=C1)S(=O)(=O)N2C(C=CC2=O)O
|
||||
| InChI |
1S/C11H11NO4S/c1-8-2-4-9(5-3-8)17(15,16)12-10(13)6-7-11(12)14/h2-7,10,13H,1H3
|
||||
| InChIKey |
LASNBXWNXQRYSO-UHFFFAOYSA-N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Carbonic anhydrase I | Target Info | Inhibitor | [1] | |
| Carbonic anhydrase II | Target Info | Inhibitor | [1] | ||
| KEGG Pathway | Nitrogen metabolismhsa00910:Nitrogen metabolism | ||||
| Proximal tubule bicarbonate reclamation | |||||
| Collecting duct acid secretion | |||||
| Gastric acid secretion | |||||
| Pancreatic secretion | |||||
| Bile secretion | |||||
| NetPath Pathway | IL4 Signaling Pathway | ||||
| EGFR1 Signaling Pathway | |||||
| Pathway Interaction Database | C-MYB transcription factor network | ||||
| PathWhiz Pathway | Gastric Acid Production | ||||
| Reactome | Erythrocytes take up carbon dioxide and release oxygen | ||||
| Erythrocytes take up oxygen and release carbon dioxide | |||||
| Reversible hydration of carbon dioxideR-HSA-1237044:Erythrocytes take up carbon dioxide and release oxygen | |||||
| Reversible hydration of carbon dioxide | |||||
| WikiPathways | Reversible Hydration of Carbon Dioxide | ||||
| Uptake of Carbon Dioxide and Release of Oxygen by Erythrocytes | |||||
| Uptake of Oxygen and Release of Carbon Dioxide by ErythrocytesWP2770:Reversible Hydration of Carbon Dioxide | |||||
| Uptake of Oxygen and Release of Carbon Dioxide by Erythrocytes | |||||
| References | |||||
| REF 1 | Bioorg Med Chem. 2010 Jun 15;18(12):4468-74. Epub 2010 May 28.A novel and one-pot synthesis of new 1-tosyl pyrrol-2-one derivatives and analysis of carbonic anhydrase inhibitory potencies. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

