Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D07UXP
|
||||
| Former ID |
DAP000999
|
||||
| Drug Name |
Cinoxacin
|
||||
| Synonyms |
Cinobac; Cinobactin; Cinoxacine; Cinoxacino; Cinoxacinum; Cinx; Clinoxacin; Uronorm; Azolinic Acid; C 8645; Lilly 64716; TNP00246; Cinobac (TN); Cinoxacine [INN-French]; Cinoxacino [INN-Spanish]; Cinoxacinum [INN-Latin]; Cinoxacin (JAN/USP/INN); Cinoxacin [USAN:BAN:INN:JAN]; 1-Ethyl-1,4-dihydro-4-oxo(1,3)dioxolo(4,5-g)cinnoline-3-carboxylic acid; 1-Ethyl-1,4-dihydro-4-oxo[1,3]dioxolo[4,5-g]cinnoline-3-carboxylic acid; 1-Ethyl-4-oxo-1,4-dihydro[1,3]dioxolo[4,5-g]cinnoline-3-carboxylic acid; 1-Ethyl-6,7-methylenedioxy-4(1H)-oxocinnoline-3-carboxylic acid; 1-ethyl-4-oxo-[1,3]dioxolo[4,5-g]cinnoline-3-carboxylic acid
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Urinary tract infections [ICD9: 599; ICD10:N39.0] | Approved | [1], [2] | ||
| Therapeutic Class |
Antiinfective Agents
|
||||
| Structure |
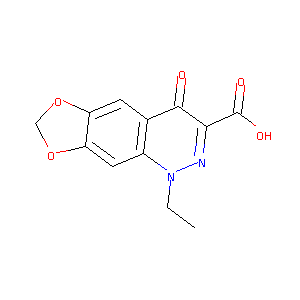
|
Download2D MOL |
|||
| Formula |
C12H10N2O5
|
||||
| Canonical SMILES |
CCN1C2=CC3=C(C=C2C(=O)C(=N1)C(=O)O)OCO3
|
||||
| InChI |
1S/C12H10N2O5/c1-2-14-7-4-9-8(18-5-19-9)3-6(7)11(15)10(13-14)12(16)17/h3-4H,2,5H2,1H3,(H,16,17)
|
||||
| InChIKey |
VDUWPHTZYNWKRN-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 28657-80-9
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
10252, 453513, 640098, 855948, 4816526, 7847937, 7978949, 8149259, 8151783, 10321339, 11110995, 11110996, 11110997, 11335493, 11360732, 11363046, 11365608, 11368170, 11371363, 11373957, 11376332, 11461704, 11466808, 11467928, 11484096, 11486535, 11488386, 11490168, 11492104, 11493966, 12015380, 15490737, 17404872, 24278343, 26611663, 26679855, 26747051, 26747052, 29221917, 46507547, 47216687, 47291046, 47440155, 47810655, 48035014, 48035015, 48110366, 48259141, 48334399, 48415782
|
||||
| SuperDrug ATC ID |
J01MB06
|
||||
| SuperDrug CAS ID |
cas=028657809
|
||||
| Target and Pathway | |||||
| Target(s) | DNA topoisomerase II | Target Info | Inhibitor | [3] | |
| References | |||||
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 018067. | ||||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
| REF 3 | The interaction and transport of beta-lactam antibiotics with the cloned rat renal organic anion transporter 1. J Pharmacol Exp Ther. 1999 Aug;290(2):672-7. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

