| Drug General Information |
| Drug ID |
D08KIB
|
| Former ID |
DIB012830
|
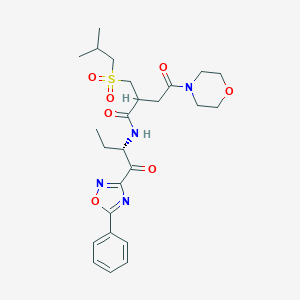
| Drug Name |
SAR-114137
|
| Indication |
Pain [ICD9: 338, 356.0, 356.8,780; ICD10:G64, G90.0, R52, G89]
|
Phase 1 |
[1]
|
|---|
| Company |
Sanofi
|
| Structure |
|
Download
2D MOL
3D MOL
|
| Target and Pathway |
| Target(s) |
Cathepsin S |
Target Info |
Inhibitor |
[1]
|
|---|
| Cathepsin K |
Target Info |
Inhibitor |
[1]
|
|
KEGG Pathway
|
Lysosome
|
|
Phagosome
|
|
Antigen processing and presentation
|
|
Tuberculosishsa04142:Lysosome
|
|
Osteoclast differentiation
|
|
Toll-like receptor signaling pathway
|
|
Rheumatoid arthritis
|
|
NetPath Pathway
|
Leptin Signaling Pathway
|
|
IL2 Signaling PathwayNetPath_7:TGF_beta_Receptor Signaling Pathway
|
|
RANKL Signaling Pathway
|
|
IL2 Signaling Pathway
|
|
Reactome
|
Endosomal/Vacuolar pathway
|
|
Degradation of the extracellular matrix
|
|
Trafficking and processing of endosomal TLR
|
|
Assembly of collagen fibrils and other multimeric structures
|
|
MHC class II antigen presentationR-HSA-1442490:Collagen degradation
|
|
Activation of Matrix Metalloproteinases
|
|
MHC class II antigen presentation
|
|
WikiPathways
|
Class I MHC mediated antigen processing & presentation
|
|
Trafficking and processing of endosomal TLRWP2018:RANKL/RANK Signaling Pathway
|
|
Osteoclast Signaling
|
| References |
| REF 1 | From laboratory to pilot plant: the solid-state process development of a highly potent cathepsin S/K inhibitor. Eur J Pharm Biopharm. 2013 Apr;83(3):436-48. |
|---|