| Drug General Information |
| Drug ID |
D08OUV
|
| Former ID |
DIB012797
|
| Drug Name |
CHF-4227
|
| Synonyms |
CHF-3316; CHF-4056; SERMs, Chiesi; Selective estrogen receptor modulators, Chiesi; CHF-3316.01
|
| Drug Type |
Small molecular drug
|
| Indication |
Osteoporosis [ICD9: 733.0, V07.4; ICD10:M80-M81, Z79.890]
|
Phase 1 |
[1]
|
|---|
| Company |
Chiesi Farmaceutici SpA
|
| Structure |
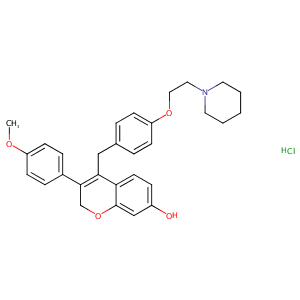
|
Download
2D MOL
3D MOL
|
| Formula |
C30H33NO4
|
| Canonical SMILES |
C1(=C(COc2c1ccc(c2)O)c1ccc(cc1)OC)Cc1ccc(cc1)OCCN1CCCCC<br />1.Cl
|
| PubChem Compound ID |
|
| Target and Pathway |
| Target(s) |
Estrogen receptor |
Target Info |
Modulator |
[2]
|
|---|
|
KEGG Pathway
|
Estrogen signaling pathway
|
|
Prolactin signaling pathway
|
|
Thyroid hormone signaling pathway
|
|
Endocrine and other factor-regulated calcium reabsorption
|
|
Proteoglycans in cancer
|
|
NetPath Pathway
|
FSH Signaling Pathway
|
|
EGFR1 Signaling Pathway
|
|
RANKL Signaling Pathway
|
|
Pathway Interaction Database
|
Regulation of nuclear SMAD2/3 signaling
|
|
Signaling events mediated by HDAC Class II
|
|
Plasma membrane estrogen receptor signaling
|
|
LKB1 signaling events
|
|
Regulation of Telomerase
|
|
ATF-2 transcription factor network
|
|
AP-1 transcription factor network
|
|
FOXM1 transcription factor network
|
|
Validated nuclear estrogen receptor alpha network
|
|
Signaling mediated by p38-alpha and p38-beta
|
|
FOXA1 transcription factor network
|
|
Reactome
|
Nuclear signaling by ERBB4
|
|
Nuclear Receptor transcription pathway
|
|
WikiPathways
|
Estrogen signaling pathway
|
|
Nuclear Receptors Meta-Pathway
|
|
Estrogen Receptor Pathway
|
|
Signaling by ERBB4
|
|
JAK/STAT
|
|
Integrated Pancreatic Cancer Pathway
|
|
Leptin signaling pathway
|
|
miR-targeted genes in muscle cell - TarBase
|
|
Integrated Breast Cancer Pathway
|
|
Nuclear Receptors
|
| References |
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800016760) |
|---|
| REF 2 | Pharmacological actions of a novel, potent, tissue-selective benzopyran estrogen. J Pharmacol Exp Ther. 2002 Oct;303(1):196-203. |
|---|