Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D08TRG
|
||||
| Former ID |
DIB014163
|
||||
| Drug Name |
AZD-3241
|
||||
| Synonyms |
Parkinson's disease therapeutic, AstraZeneca
|
||||
| Indication | Parkinson's disease [ICD9: 332; ICD10:G20] | Phase 2 | [525118] | ||
| Company |
AstraZeneca plc
|
||||
| Structure |
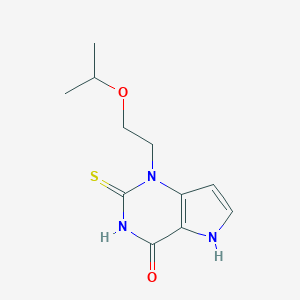
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Myeloperoxidase | Target Info | Inhibitor | [533278] | |
| KEGG Pathway | Phagosome | ||||
| Transcriptional misregulation in cancer | |||||
| Pathway Interaction Database | C-MYB transcription factor network | ||||
| IL23-mediated signaling events | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

