Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D08YDT
|
||||
| Former ID |
DIB009576
|
||||
| Drug Name |
KAI-4169
|
||||
| Synonyms |
Protein kinase C modulator (secondary hyperparathyroidism), KAI pharmaceuticals; Calcium-sensing receptor agonist (secondary hyperparathyroidism), KAI Pharmaceuticals
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Hyperparathyroidism [ICD9: 252.02, 588.81; ICD10:E21.1] | Phase 2 | [523864] | ||
| Company |
KAI Pharmaceuticals Inc
|
||||
| Structure |
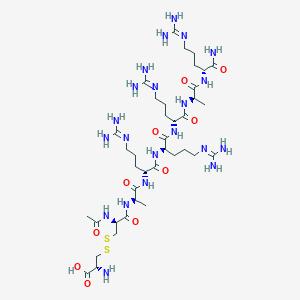
|
Download2D MOL |
|||
| Formula |
C38H73N21O10S2
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| Target and Pathway | |||||
| Target(s) | Extracellular calcium-sensing receptor | Target Info | Agonist | [532354] | |
| Pathway Interaction Database | E-cadherin signaling in keratinocytes | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

