Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D09JXH
|
||||
| Former ID |
DIB008576
|
||||
| Drug Name |
LUM001
|
||||
| Synonyms |
SHP625
|
||||
| Indication | Primary biliary cirrhosis [ICD9: 571.6; ICD10:K74.3] | Phase 2 | [525031] | ||
| Company |
Lumena pharmaceuticals
|
||||
| Structure |
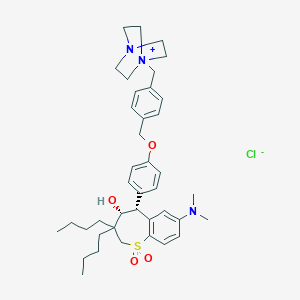
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Ileal bile acid transfer | Target Info | Inhibitor | [550354] | |
| KEGG Pathway | Bile secretion | ||||
| Reactome | Recycling of bile acids and salts | ||||
| WikiPathways | Bile acid and bile salt metabolism | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

