Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0AF3A
|
||||
| Former ID |
DNC001154
|
||||
| Drug Name |
Protoporphyrin IX
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Discovery agent | Investigative | [536585] | ||
| Structure |
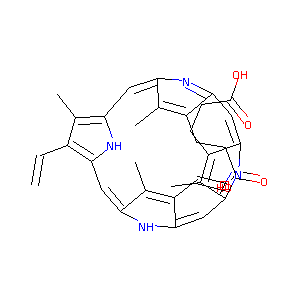
|
Download2D MOL |
|||
| Formula |
C34H34N4O4
|
||||
| Canonical SMILES |
CC1=C(C2=CC3=C(C(=C(N3)C=C4C(=C(C(=N4)C=C5C(=C(C(=N5)C=<br />C1N2)C)CCC(=O)O)CCC(=O)O)C)C=C)C)C=C
|
||||
| InChI |
1S/C34H34N4O4/c1-7-21-17(3)25-13-26-19(5)23(9-11-33(39)40)31(37-26)16-32-24(10-12-34(41)42)20(6)28(38-32)15-30-22(8-2)18(4)27(36-30)14-29(21)35-25/h7-8,13-16,35-36H,1-2,9-12H2,3-6H3,(H,39,40)(H,41,42)
|
||||
| InChIKey |
ZCFFYALKHPIRKJ-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 15415-30-2
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
5261, 69254, 596306, 841432, 4413024, 4903491, 7640482, 7890005, 8137800, 8143177, 8149710, 10526884, 11111672, 11335249, 11360488, 11364194, 11366756, 11369318, 11372499, 11373857, 11377480, 11461460, 11484907, 11488832, 11491255, 11492234, 11495114, 11537733, 14788515, 14837581, 17405531, 24278655, 26612168, 26681019, 26747639, 26747640, 26750425, 29224047, 32963314, 32964437, 46171309, 46506247, 47193689, 47217031, 47365444, 47811014, 48035384, 49748473, 50070313, 50071311
|
||||
| ChEBI ID |
ChEBI:15430
|
||||
| SuperDrug ATC ID |
B06AB01
|
||||
| SuperDrug CAS ID |
cas=015489904
|
||||
| Target and Pathway | |||||
| Target(s) | Glutathione S-transferase | Target Info | Inhibitor | [536585] | |
| BioCyc Pathway | C20 prostanoid biosynthesis | ||||
| KEGG Pathway | Arachidonic acid metabolism | ||||
| Metabolic pathways | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

