Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0AH6V
|
||||
| Former ID |
DIB007452
|
||||
| Drug Name |
BN-2629
|
||||
| Synonyms |
SG-2000; SJG-136; SP-2001
|
||||
| Indication | Solid tumours [ICD9: 140-199, 210-229; ICD10:C00-D48] | Phase 1/2 | [1] | ||
| Company |
Spirogen Ltd
|
||||
| Structure |
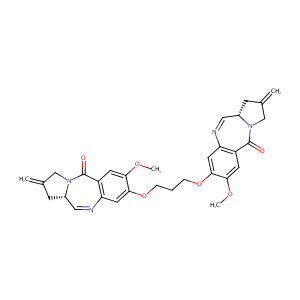
|
Download2D MOL |
|||
| Canonical SMILES |
C1(=O)N2[C@H](C=Nc3c1cc(c(c3)OCCCOc1cc3c(C(=O)N4[C@H](C<br />=N3)CC(=C)C4)cc1OC)OC)CC(=C)C2
|
||||
| CAS Number |
CAS 232931-57-6
|
||||
| Target and Pathway | |||||
| Target(s) | Human DNA | Target Info | Binder | [2] | |
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT02034227) Safety, Tolerability Study of SG2000 in the Treatment of Advanced Chronic Lymphocytic Leukemia and Acute Myeloid Leukemia. U.S. National Institutes of Health. | ||||
| REF 2 | Single-Nucleotide Polymorphisms in Rv2629 Are Specific for Mycobacterium tuberculosis Genotypes Beijing and Ghana but Not Associated with Rifampin Resistance . J Clin Microbiol. 2009 January; 47(1): 223-226. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

