Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0C6DT
|
||||
| Former ID |
DAP000726
|
||||
| Drug Name |
Esomeprazole
|
||||
| Synonyms |
AULCER; Antra; Audazol; Axagon; Belmazol; Ceprandal; Danlox; Demeprazol; Desec; Dizprazol; Dudencer; Elgam; Emeproton; Emilok; Epirazole; Erbolin; Esomeprazol; Esomeprazolum; Esomperazole; Esopral; Exter; Gasec; Gastrimut; Gastroloc; Gibancer; Indurgan; Inhibitron; Inhipump; Lensor; Logastric; Lomac; Losec; Lucen; Mepral; Miol; Miracid; Mopral; Morecon; Nexiam; Nexium; Nilsec; Nopramin; Nuclosina; OMEP; OMZ; Ocid; Olexin; Olit; Omapren; Omebeta; Omed; Omegast; Omepradex; Omepral; Omeprazol; Omeprazole; Omeprazolum; Omeprazon; Omeprazone; Omeprol; Omesek; Omez; Omezol; Omezolan; Omid; Omisec; Omizac; Ompanyt; Ortanol; Osiren; Ozoken; Paprazol; Parizac; Pepticum; Pepticus; Peptilcer; Prazentol; Prazidec; Prazolit; Prilosec; Procelac; Proclor; Prysma; Ramezol; Regulacid; Result; Sanamidol; Secrepina; Ulceral; Ulcesep; Ulcometion; Ulcozol; Ulcsep; Ulsen; Ultop; Ulzol; Victrix; Zefxon; Zegerid; Zepral; Zimor; Zoltum; Antra MUPS; Inexium paranova; Nexium IV; Omeprazole magnesium; Prilosec OTC; Tedec Ulceral; O0359; Omebeta 20; AGI-010; Axagon (TN); DM-3458; Esomeprazole (INN); Esomeprazole [INN:BAN]; Esopral (TN); H 168-68; H 168/68; Inexium paranova (TN); Lucen (TN); Nexiam (TN); Nexium (TN); Omeprazol [INN-Spanish]; Omeprazole delayed-release; Omeprazolum [INN-Latin]; Prilosec (TN); SAN-15; Sompraz (TN); Zoleri (TN); H-168/68; Omeprazole (JAN/USP/INN); Omeprazole [USAN:INN:BAN:JAN]; Losec, Omesec, Prilosec, Zegerid, Omeprazole; (-)-Omeprazole; (S)-(-)-Omeprazole; (S)-Omeprazole; 2-(((3,5-Dimethyl-4-methoxy-2-pyridyl)methyl)sulfinyl)-5-methoxy-1H-benzimidazole; 2-({[3,5-dimethyl-4-(methyloxy)pyridin-2-yl]methyl}sulfinyl)-5-(methyloxy)-1H-benzimidazole; 5-Methoxy-2-(((4-methoxy-3,5-dimethyl-2-pyridyl)methyl)sulfinyl)benzimidazole; 5-Methoxy-2-((S)-((4-methoxy-3,5-dimethyl-2-pyridyl)methyl)sulfinyl)benzimidazole; 5-Methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole; 5-Methoxy-2[(4-methoxy-3,5-dimethyl-2-pyridyl)methylsulfinyl]-1H-benzimidazole; 5-methoxy-2-{(S)-[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfinyl}-1H-benzimidazole; 5-methoxy-2-{[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfinyl}-1-methyl-1H-benzimidazole; 5-methoxy-2-{[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfinyl}-1H-benzimidazole; 6-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methylsulfinyl]-1H-benzimidazole; 6-methoxy-2-{[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfinyl}-1H-benzimidazole
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Antiulcer Agents
|
||||
| Company |
AstraZeneca
|
||||
| Structure |
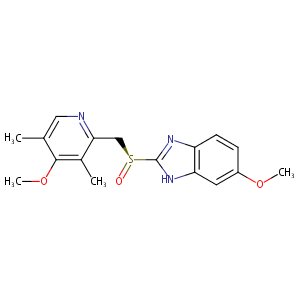
|
Download2D MOL |
|||
| Formula |
C17H19N3O3S
|
||||
| InChI |
InChI=1S/C17H19N3O3S/c1-10-8-18-15(11(2)16(10)23-4)9-24(21)17-19-13-6-5-12(22-3)7-14(13)20-17/h5-8H,9H2,1-4H3,(H,19,20)/t24-/m0/s1
|
||||
| InChIKey |
SUBDBMMJDZJVOS-DEOSSOPVSA-N
|
||||
| CAS Number |
CAS 161796-78-7
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
591076, 14875947, 14900571, 44707139, 56394969, 80295315, 93166178, 96024613, 124759673, 124968856, 126525308, 126670961, 129288902, 129288903, 137001228, 137752931, 139533883, 152040240, 160825217, 163092495, 163384365, 163840367, 164044845, 175267781, 175269116, 175437905, 178102135, 189657728, 198993898, 204429634, 211535433, 223971225, 226409261, 246378786, 249855831, 250200763, 251886328, 251964001, 252074449, 252430137
|
||||
| ChEBI ID |
ChEBI:50275
|
||||
| SuperDrug ATC ID |
A02BC05
|
||||
| SuperDrug CAS ID |
cas=161973100
|
||||
| Target and Pathway | |||||
| Target(s) | H+ K+ ATPase | Target Info | Modulator | [556264] | |
| References | |||||
| Ref 536779 | Esomeprazole: a review of its use in the management of gastric acid-related diseases in adults. Drugs. 2008;68(11):1571-607. | ||||
| Ref 540887 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5488). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

