Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0D3TX
|
||||
| Former ID |
DNCL001975
|
||||
| Drug Name |
EVP-6124
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Mild to moderate alzheimer disease; Schizophrenia [ICD9: 331, 295; ICD10:G30, F20] | Phase 3 | [1] | ||
| Company |
EnVivo Pharmaceuticals
|
||||
| Structure |
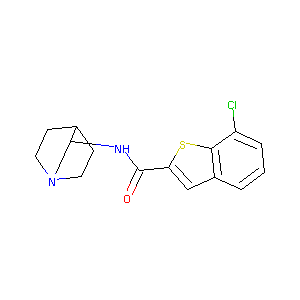
|
Download2D MOL |
|||
| Formula |
C16H17ClN2OS
|
||||
| Canonical SMILES |
C1CN2CCC1C(C2)NC(=O)C3=CC4=C(S3)C(=CC=C4)Cl
|
||||
| InChI |
1S/C16H17ClN2OS/c17-12-3-1-2-11-8-14(21-15(11)12)16(20)18-13-9-19-6-4-10(13)5-7-19/h1-3,8,10,13H,4-7,9H2,(H,18,20)/t13-/m0/s1
|
||||
| InChIKey |
SSRDSYXGYPJKRR-ZDUSSCGKSA-N
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| Target and Pathway | |||||
| Target(s) | Neuronal acetylcholine receptor protein, alpha-7 chain | Target Info | Modulator | [2] | |
| KEGG Pathway | Calcium signaling pathway | ||||
| Neuroactive ligand-receptor interaction | |||||
| Cholinergic synapse | |||||
| Nicotine addiction | |||||
| Chemical carcinogenesis | |||||
| PANTHER Pathway | Alzheimer disease-amyloid secretase pathway | ||||
| Nicotinic acetylcholine receptor signaling pathway | |||||
| Reactome | Highly calcium permeable postsynaptic nicotinic acetylcholine receptors | ||||
| WikiPathways | SIDS Susceptibility Pathways | ||||
| Neurotransmitter Receptor Binding And Downstream Transmission In The Postsynaptic Cell | |||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT02004392) Study of the Safety and Clinical Effects of 2 Doses of EVP-6124 in Subjects With Alzheimer's Disease Who Complete Study EVP-6124-024 or EVP-6124-025. U.S. National Institutes of Health. | ||||
| REF 2 | Normalizing effects of EVP-6124, an alpha-7 nicotinic partial agonist, on event-related potentials and cognition: a proof of concept, randomized trial in patients with schizophrenia. J Psychiatr Pract. 2014 Jan;20(1):12-24. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

