Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0E6SX
|
||||
| Former ID |
DIB001038
|
||||
| Drug Name |
AZD-5069
|
||||
| Synonyms |
CXCR2 inhibitor (COPD), Astrazeneca
|
||||
| Indication | Chronic obstructive pulmonary disease [ICD9: 490-492, 494-496; ICD10:J40-J44, J47] | Discontinued in Phase 1 | [548946] | ||
| Company |
AstraZeneca plc
|
||||
| Structure |
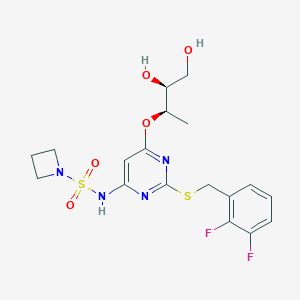
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | High affinity interleukin-8 receptor B | Target Info | Inhibitor | [533162] | |
| NetPath Pathway | TNFalpha Signaling Pathway | ||||
| Pathway Interaction Database | IL8- and CXCR2-mediated signaling events | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

