Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0E9TP
|
||||
| Former ID |
DAP000866
|
||||
| Drug Name |
Sulodexide
|
||||
| Indication | Central or sensorineural tinnitus [ICD9: 388.3; ICD10:H93.1] | Approved | [536297] | ||
| Therapeutic Class |
Antithrombotic Agents
|
||||
| Structure |
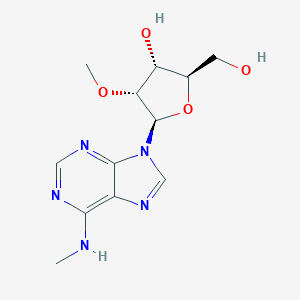
|
Download2D MOL |
|||
| CAS Number |
CAS 57821-29-1
|
||||
| SuperDrug ATC ID |
B01AB11
|
||||
| Target and Pathway | |||||
| Target(s) | Antithrombin-III | Target Info | Activator | [537594], [538076] | |
| KEGG Pathway | Complement and coagulation cascades | ||||
| PANTHER Pathway | Blood coagulation | ||||
| Pathway Interaction Database | Glypican 1 network | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

