Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0EV6T
|
||||
| Former ID |
DAP001085
|
||||
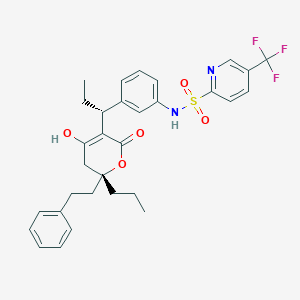
| Drug Name |
Tipranavir
|
||||
| Synonyms |
Aptivus; TPV; PNU 140690; U 140690; Aptivus (Boehringer Ingelheim); Aptivus (TN); Aptivus(TM); PNU-140690; PNU-140690E; Tipranavir (INN); U-140690; N-[3-[(1R)-1-[(2R)-6-hydroxy-4-oxo-2-phenethyl-2-propyl-3H-pyran-5-yl]propyl]phenyl]-5-(trifluoromethyl)pyridine-2-sulfonamide; N-(3-{(1R)-1-[(6R)-4-HYDROXY-2-OXO-6-PHENETHYL-6-PROPYL-5,6-DIHYDRO-2H-PYRAN-3-YL]PROPYL}PHENYL)-5-(TRIFLUOROMETHYL)-2-PYRIDINESULFONAMIDE; TPV
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Human immunodeficiency virus infection [ICD9: 279.3; ICD10:B20-B26] | Approved | [1] | ||
| Therapeutic Class |
Anti-HIV Agents
|
||||
| Company |
Boehriger Ingelheim
|
||||
| Structure |
|
Download2D MOL |
|||
| Formula |
C31H33F3N2O5S
|
||||
| CAS Number |
CAS 174484-41-4
|
||||
| PubChem Compound ID | |||||
| SuperDrug ATC ID |
J05AE09
|
||||
| Drug Resistance Mutation (DRM) | |||||
| DRM | DRM Info | ||||
| Target and Pathway | |||||
| Target(s) | HIV protease | Target Info | Modulator | [2] | |
| References | |||||
| REF 1 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. Epub 2007 Feb 20. | ||||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

