Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0F2JY
|
||||
| Former ID |
DIB016972
|
||||
| Drug Name |
LY-2334737
|
||||
| Synonyms |
Gem prodrug (cancer), Eli Lilly; Gemcitabine prodrug (cancer), Lilly
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Solid tumours [ICD9: 140-199, 210-229; ICD10:C00-D48] | Phase 1 | [1] | ||
| Company |
Eli Lilly & Co
|
||||
| Structure |
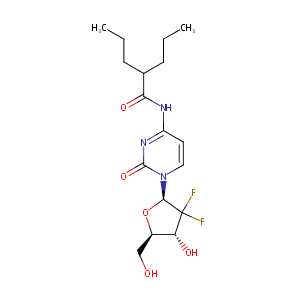
|
Download2D MOL |
|||
| Formula |
C17H25F2N3O5
|
||||
| Canonical SMILES |
C1(F)(F)[C@H](n2ccc(nc2=O)NC(=O)C(CCC)CCC)O[C@@H]([C@H]<br />1O)CO
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Ribonucleoside-diphosphatereductase subunit M2 | Target Info | Modulator | [2] | |
| Human DNA | Target Info | Modulator | [2] | ||
| BioCyc Pathway | Pyrimidine deoxyribonucleotides biosynthesis from CTP | ||||
| Pyrimidine deoxyribonucleotides de novo biosynthesis | |||||
| Guanosine nucleotides de novo biosynthesis | |||||
| Superpathway of pyrimidine deoxyribonucleotides de novo biosynthesis | |||||
| Superpathway of purine nucleotide salvage | |||||
| Purine nucleotides de novo biosynthesis | |||||
| Adenosine deoxyribonucleotides de novo biosynthesis | |||||
| Guanosine deoxyribonucleotides de novo biosynthesis | |||||
| KEGG Pathway | Purine metabolism | ||||
| Pyrimidine metabolism | |||||
| Glutathione metabolism | |||||
| Metabolic pathways | |||||
| p53 signaling pathway | |||||
| NetPath Pathway | EGFR1 Signaling Pathway | ||||
| PANTHER Pathway | p53 pathway | ||||
| De novo purine biosynthesis | |||||
| De novo pyrimidine deoxyribonucleotide biosynthesis | |||||
| Pathway Interaction Database | E2F transcription factor network | ||||
| PathWhiz Pathway | Purine Metabolism | ||||
| Pyrimidine Metabolism | |||||
| Reactome | E2F mediated regulation of DNA replication | ||||
| G1/S-Specific Transcription | |||||
| WikiPathways | Nucleotide Metabolism | ||||
| Retinoblastoma (RB) in Cancer | |||||
| Metabolism of nucleotides | |||||
| Fluoropyrimidine Activity | |||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT01648764) A Study of LY2334737 in Participants With Cancer That is Advanced and/or Has Spread. U.S. National Institutes of Health. | ||||
| REF 2 | Phase I study of oral gemcitabine prodrug (LY2334737) in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2013 Jun;71(6):1645-55. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

