Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0F3PU
|
||||
| Former ID |
DCL000535
|
||||
| Drug Name |
Iboctadekin + Doxil
|
||||
| Indication | Ovarian cancer [ICD9: 183; ICD10:C56] | Phase 1 | [1], [2] | ||
| Company |
GSK
|
||||
| Structure |
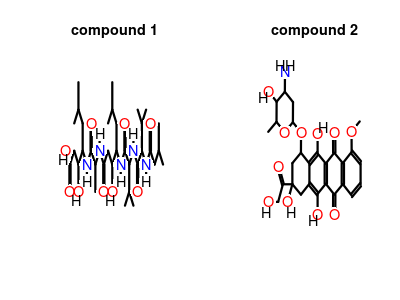
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | DNA topoisomerase II | Target Info | Inhibitor | [3] | |
| Interleukin-18 | Target Info | Modulator | [3] | ||
| KEGG Pathway | Cytokine-cytokine receptor interaction | ||||
| NOD-like receptor signaling pathway | |||||
| Cytosolic DNA-sensing pathway | |||||
| Salmonella infection | |||||
| Legionellosis | |||||
| African trypanosomiasis | |||||
| Malaria | |||||
| Tuberculosis | |||||
| Influenza A | |||||
| Inflammatory bowel disease (IBD) | |||||
| Rheumatoid arthritis | |||||
| NetPath Pathway | IL4 Signaling Pathway | ||||
| PANTHER Pathway | Interleukin signaling pathway | ||||
| Toll receptor signaling pathway | |||||
| Pathway Interaction Database | IL27-mediated signaling events | ||||
| IL12-mediated signaling events | |||||
| IL23-mediated signaling events | |||||
| Cellular roles of Anthrax toxin | |||||
| IL12 signaling mediated by STAT4 | |||||
| Reactome | Interleukin-1 processing | ||||
| WikiPathways | Hypertrophy Model | ||||
| IL1 and megakaryotyces in obesity | |||||
| Corticotropin-releasing hormone | |||||
| NOD pathway | |||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT00659178) Combination Study Of SB-485232 (Interleukin 18) And Doxil For Advanced Stage Epithelial Ovarian Cancer. U.S. National Institutes of Health. | ||||
| REF 2 | Clinical pipeline report, company report or official report of GlaxoSmithKline. | ||||
| REF 3 | Clinical pipeline report, company report or official report of GlaxoSmithKline (2009). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

