Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0F4JK
|
||||
| Former ID |
DIB011432
|
||||
| Drug Name |
CE-224535
|
||||
| Synonyms |
CP-23575
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Arthralgia [ICD10:M25.5] | Phase 2/3 | [1] | ||
| Company |
Pfizer Inc
|
||||
| Structure |
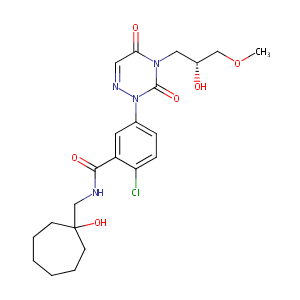
|
Download2D MOL |
|||
| Formula |
C22H29ClN4O6
|
||||
| Canonical SMILES |
C1CCCCC(C1)(O)CNC(=O)c1c(ccc(c1)n1ncc(=O)n(c1=O)C[C@H](<br />COC)O)Cl
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | P2X purinoceptor 7 | Target Info | Antagonist | [2] | |
| KEGG Pathway | Calcium signaling pathway | ||||
| Neuroactive ligand-receptor interaction | |||||
| Reactome | The NLRP3 inflammasome | ||||
| WikiPathways | Nucleotide-binding domain, leucine rich repeat containing receptor (NLR) signaling pathways | ||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT00628095) Study of CE-224,535 A Twice Daily Pill To Control Rheumatoid Arthritis In Patients Who Have Not Totally Improved With Methotrexate. U.S. National Institutes of Health. | ||||
| REF 2 | Efficacy and safety of CE-224,535, an antagonist of P2X7 receptor, in treatment of patients with rheumatoid arthritis inadequately controlled by methotrexate. J Rheumatol. 2012 Apr;39(4):720-7. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

