Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0G3DL
|
||||
| Former ID |
DAP000197
|
||||
| Drug Name |
Rifampin
|
||||
| Synonyms |
Abrifam; Archidyn; Arficin; Arzide; Benemicin; Benemycin; Dipicin; Doloresum; Eremfat; Famcin; Fenampicin; RFP; RMP; Ramp; Rifa; Rifadin; Rifadine; Rifagen; Rifaldazin; Rifaldazine; Rifaldin; Rifam; Rifamor; Rifampicin; Rifampicina; Rifampicine; Rifampicinum; Rifamsolin; Rifaprodin; Rifcin; Rifinah; Rifobac; Rifoldin; Rifoldine; Riforal; Rimactan; Rimactane; Rimactazid; Rimactizid; Rimazid; Rimycin; Sinerdol; Tubocin; Rifamicin AMP; Rifampicin SV; Rifampicine [French]; Rifampin [USAN]; Rifamycin AMP; Ba 41166; AZT + Rifampin; BA-41166E; Ba 41166/E; DRG-0109; Dione 21-acetate; L-5103; L-5103 Lepetit; Piperine & Rifampicin; R-Cin; R/AMP; Reserpine & Rifampicin; Rifadin (TN); Rifadin I.V; Rifampicin & EEP; Rifampicin & Propolis; Rifampicina [INN-Spanish]; Rifampicinum [INN-Latin]; Rifampin (USP); Rimactan (TN); Rimactane (TN); Rimycin (TN); Sinerdol (TN); Tubocin (TN); Rifadin I.V.; Rifampicin (JP15/INN); Rifampicin[INN:BAN:JAN]; Rifadin, Rimactane, Rifampicin, Rifampin; 1-b]furan-21-yl acetate; 3-(((4-Methyl-1-piperazinyl)imino)-methyl)rifamycin; 3-(((4-Methyl-1-piperazinyl)imino)methyl)rifamycin SV; 3-(4-Methylpiperazinyliminomethyl)-rifamycin SV; 3-(4-Methylpiperazinyliminomethyl)rifamycin SV; 3-([(4-Methyl-1-piperazinyl)imino]methyl)rifamycin SV; 3-[(4-Methyl-1-piperazinyl)iminomethyl]rifamycin SV; 3-[[(4-Methyl-1-piperazinyl)imino]-methyl]rifamycin; 8-(((4-Methyl-1-piperazinyl)imino)methyl)rifamycin SV; 8-(4-Methylpiperazinyliminomethyl) rifamycin SV; 8-[[(4-Methyl-1-piperazinyl)imino[methyl]rifamycin; 8-[[(4-Methyl-1-piperazinyl)imino]methyl]rifamycin sv; 8-[[(4-Methylpiperazinyl)imino]methyl]rifamycin sv; 8CI)
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Tuberculosis [ICD9: 010-018; ICD10:A15-A19] | Approved | [536923] | ||
| Therapeutic Class |
Antituberculosis Agents
|
||||
| Company |
Norvatis Phamaceuticals Corporation
|
||||
| Structure |
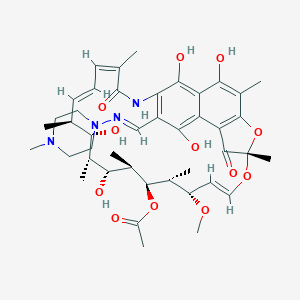
|
Download2D MOL |
|||
| Formula |
C43H58N4O12
|
||||
| Canonical SMILES |
CC1C=CC=C(C(=O)NC2=C(C3=C(C(=C4C(=C3C(=O)C2=CNN5CCN(CC5<br />)C)C(=O)C(O4)(OC=CC(C(C(C(C(C(C1O)C)O)C)OC(=O)C)C)OC)C)<br />C)O)O)C
|
||||
| InChI |
1S/C43H58N4O12/c1-21-12-11-13-22(2)42(55)45-33-28(20-44-47-17-15-46(9)16-18-47)37(52)30-31(38(33)53)36(51)26(6)40-32(30)41(54)43(8,59-40)57-19-14-29(56-10)23(3)39(58-27(7)48)25(5)35(50)24(4)34(21)49/h11-14,19-21,23-25,29,34-35,39,44,49-51,53H,15-18H2,1-10H3,(H,45,55)/b12-11+,19-14+,22-13-,28-20+
|
||||
| InChIKey |
FZYOVNIOYYPUPY-HEGPSGTHSA-N
|
||||
| CAS Number |
CAS 13292-46-1
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| ChEBI ID |
ChEBI:28077
|
||||
| SuperDrug ATC ID |
J04AB02
|
||||
| SuperDrug CAS ID |
cas=013292461
|
||||
| Drug Resistance Mutation (DRM) | |||||
| DRM | DRM Info | ||||
| Target and Pathway | |||||
| Target(s) | bacterial RNA polymerase switch region | Target Info | Inhibitor | [535323], [536636] | |
| References | |||||
| Ref 535323 | Bacillus subtilis tolerance of moderate concentrations of rifampin involves the sigma(B)-dependent general and multiple stress response. J Bacteriol. 2002 Jan;184(2):459-67. | ||||
| Ref 536636 | Novel molecular targets for antimalarial drug development. Chem Biol Drug Des. 2008 Apr;71(4):287-97. Epub 2008 Feb 22. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

