Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0G4DE
|
||||
| Former ID |
DNC000313
|
||||
| Drug Name |
BIA 3-202
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Parkinson's disease [ICD9: 332; ICD10:G20] | Phase 2 | [1] | ||
| Structure |
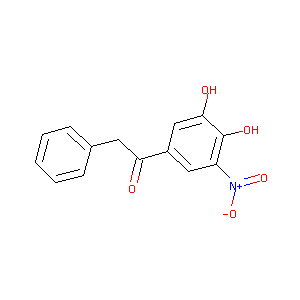
|
Download2D MOL |
|||
| Formula |
C14H11NO5
|
||||
| Canonical SMILES |
C1=CC=C(C=C1)CC(=O)C2=CC(=C(C(=C2)O)O)[N+](=O)[O-]
|
||||
| InChI |
1S/C14H11NO5/c16-12(6-9-4-2-1-3-5-9)10-7-11(15(19)20)14(18)13(17)8-10/h1-5,7-8,17-18H,6H2
|
||||
| InChIKey |
MRFOLGFFTUGAEB-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 274925-86-9
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| Target and Pathway | |||||
| Target(s) | Catechol-O-methyl-transferase | Target Info | Inhibitor | [2] | |
| BioCyc Pathway | L-dopa degradation | ||||
| Dopamine degradation | |||||
| Noradrenaline and adrenaline degradation | |||||
| KEGG Pathway | Steroid hormone biosynthesis | ||||
| Tyrosine metabolism | |||||
| Metabolic pathways | |||||
| Dopaminergic synapse | |||||
| PANTHER Pathway | Adrenaline and noradrenaline biosynthesis | ||||
| Dopamine receptor mediated signaling pathway | |||||
| PathWhiz Pathway | Tyrosine Metabolism | ||||
| WikiPathways | Methylation Pathways | ||||
| Metapathway biotransformation | |||||
| Estrogen metabolism | |||||
| Biogenic Amine Synthesis | |||||
| Dopamine metabolism | |||||
| Phase II conjugation | |||||
| Neurotransmitter Clearance In The Synaptic Cleft | |||||
| References | |||||
| REF 1 | Effects of nebicapone on levodopa pharmacokinetics, catechol-O-methyltransferase activity, and motor fluctuations in patients with Parkinson disease. Clin Neuropharmacol. 2008 Jan-Feb;31(1):2-18. | ||||
| REF 2 | Chemical synthesis and characterization of conjugates of a novel catechol-O-methyltransferase inhibitor. Bioconjug Chem. 2002 Sep-Oct;13(5):1112-8. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

