Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0GL7H
|
||||
| Former ID |
DIB001392
|
||||
| Drug Name |
Talotrexin
|
||||
| Synonyms |
Talvesta; Talotrexin ammonium; PT-523; PT-633; 10-deaza-PT-523
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Solid tumours [ICD9: 140-199, 210-229; ICD10:C00-D48] | Phase 1/2 | [521643] | ||
| Company |
Hana Biosciences; Dana-Farber Cancer Institute Inc
|
||||
| Structure |
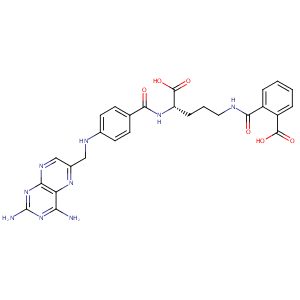
|
Download2D MOL |
|||
| Formula |
C27H27N9O6
|
||||
| Canonical SMILES |
n1c(c2c(nc1N)ncc(n2)CNc1ccc(C(=O)N[C@H](C(=O)O)CCCNC(=O<br />)c2c(C(=O)O)cccc2)cc1)N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Folate receptor alpha | Target Info | Antagonist | [544163] | |
| KEGG Pathway | Endocytosis | ||||
| WikiPathways | Folate Metabolism | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

