Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0H0ND
|
||||
| Former ID |
DAP000552
|
||||
| Drug Name |
Simvastatin
|
||||
| Synonyms |
Cholestat; Coledis; Colemin; Corolin; Denan; Labistatin; Lipex; Lipovas; Lodales; Medipo; Nivelipol; Pantok; Rendapid; Simovil; Simvastatina; Simvastatine; Simvastatinum; Sinvacor; Sivastin; Synvinolin; Vasotenal; Zocor; Zocord; Simvast CR; Simvastatina [Spanish]; Simvastatine [French]; Simvastatinum [Latin]; MK 0733; MK 733; MK733; TNP00259; DRG-0320; KS-1113; L 644128-000U; MK-0733; MK-733; Simcard (TN); Simlup (TN); Simvacor (TN); Simvastatin & Primycin; Simvastatin, Compactin; Zocor (TN); Simvastatin [USAN:INN:BAN]; Simvastatin (JAN/USP/INN); Zocor, Simlup, Simcard, Simvacor, Simvoget, Zorced, Simvastatin; [(1S,3R,7S,8S,8aR)-8-[2-[(2R,4R)-4-hydroxy-6-oxooxan-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl] 2,2-dimethylbutanoate; Butanoic acid, 2,2-dimethyl-, (1S,3R,7S,8S,8aR)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-[(2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl]ethyl]-1-naphthalenyl ester; Butanoic acid, 2,2-dimethyl-, (1S,3R,7S,8S,8aR)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-(2-((2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)ethyl)-1-naphthalenyl ester; Butanoic acid, 2,2-dimethyl-, (1S,3R,7S,8S,*aR)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-(2-((2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)ethyl)-1-naphthalenyl ester; (1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotetrahydro-2H-pyran-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl 2,2-dimethylbutanoate; Butanoic acid, 2,2-dimethyl-,1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-(tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)-ethyl]-1-naphthalenyl ester, [1S-[1 alpha,3 alpha,7 beta,8 beta(2S*,4S*),-8a beta; 2,2-Dimethylbutanoic acid (1S,3R,7S,8S,8aR)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-[(2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl]ethyl]-1-naphthalenyl ester; 2,2-Dimethylbutyric acid, 8-ester with (4R,6R)-6-(2-((1S,2S,6R,8S,8aR)-1,2,6,7,8,8a-hexahydro-8-hydroxy-2,6-dimethyl-1-naphthyl)ethyl)tetrahydro-4-hydroxy-2H-pyran-2-one
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Anticholesteremic Agents
|
||||
| Company |
Merck & Co
|
||||
| Structure |
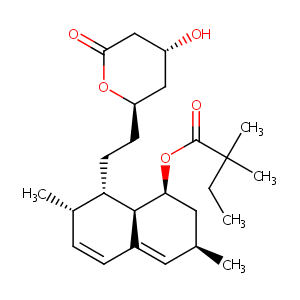
|
Download2D MOL |
|||
| Formula |
C25H38O5
|
||||
| InChI |
InChI=1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19+,20-,21-,23-/m0/s1
|
||||
| InChIKey |
RYMZZMVNJRMUDD-HGQWONQESA-N
|
||||
| CAS Number |
CAS 79902-63-9
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
496592, 648581, 7847500, 7980599, 8183649, 10321271, 10852028, 11113242, 11342166, 11362349, 11364611, 11367173, 11369735, 11371657, 11374383, 11377897, 11466893, 11468013, 11485617, 11486566, 11487751, 11489487, 11490485, 11492447, 11495531, 11528633, 11533326, 12013879, 12146013, 14831549, 14929313, 24724617, 25819951, 26612685, 26680673, 26759532, 34718442, 46508654, 47365442, 47440515, 47736737, 47885633, 47885634, 48110715, 48334759, 48416540, 49698671, 50086525, 50100555, 50100556
|
||||
| ChEBI ID |
ChEBI:9150
|
||||
| SuperDrug ATC ID |
C10AA01
|
||||
| SuperDrug CAS ID |
cas=079902639
|
||||
| Target and Pathway | |||||
| Target(s) | 3-hydroxy-3-methylglutaryl-coenzyme A reductase | Target Info | Inhibitor | [535246] | |
| PANTHER Pathway | Cholesterol biosynthesis | ||||
| PathWhiz Pathway | Steroid Biosynthesis | ||||
| References | |||||
| Ref 537114 | Emerging therapies for multiple myeloma. Expert Opin Emerg Drugs. 2009 Mar;14(1):99-127. | ||||
| Ref 539966 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2955). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

