Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0H4CF
|
||||
| Former ID |
DNC010198
|
||||
| Drug Name |
3-((4-aminophenyl)diazenyl)benzenesulfonamide
|
||||
| Synonyms |
3-(4'-Aminophenyl)diazenylbenzenesulfonamide
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Discovery agent | Investigative | [1] | ||
| Structure |
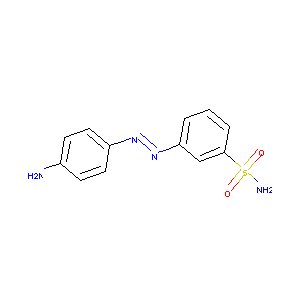
|
Download2D MOL |
|||
| Formula |
C12H12N4O2S
|
||||
| Canonical SMILES |
C1=CC(=CC(=C1)S(=O)(=O)N)N=NC2=CC=C(C=C2)N
|
||||
| InChI |
1S/C12H12N4O2S/c13-9-4-6-10(7-5-9)15-16-11-2-1-3-12(8-11)19(14,17)18/h1-8H,13H2,(H2,14,17,18)
|
||||
| InChIKey |
VAOWCMBATGAWIO-UHFFFAOYSA-N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Carbonic anhydrase IX | Target Info | Inhibitor | [1] | |
| Carbonic anhydrase XII | Target Info | Inhibitor | [1] | ||
| Carbonic anhydrase II | Target Info | Inhibitor | [2] | ||
| KEGG Pathway | Nitrogen metabolismhsa00910:Nitrogen metabolismhsa00910:Nitrogen metabolism | ||||
| Proximal tubule bicarbonate reclamation | |||||
| Collecting duct acid secretion | |||||
| Gastric acid secretion | |||||
| Pancreatic secretion | |||||
| Bile secretion | |||||
| NetPath Pathway | TGF_beta_Receptor Signaling PathwayNetPath_16:IL4 Signaling Pathway | ||||
| EGFR1 Signaling Pathway | |||||
| Pathway Interaction Database | HIF-1-alpha transcription factor network | ||||
| Reactome | Regulation of gene expression by Hypoxia-inducible Factor | ||||
| Reversible hydration of carbon dioxideR-HSA-1475029:Reversible hydration of carbon dioxideR-HSA-1237044:Erythrocytes take up carbon dioxide and release oxygen | |||||
| Erythrocytes take up oxygen and release carbon dioxide | |||||
| Reversible hydration of carbon dioxide | |||||
| WikiPathways | Vitamin D Receptor Pathway | ||||
| Reversible Hydration of Carbon Dioxide | |||||
| Regulation of Hypoxia-inducible Factor (HIF) by OxygenWP2770:Reversible Hydration of Carbon Dioxide | |||||
| miR-targeted genes in muscle cell - TarBase | |||||
| miR-targeted genes in leukocytes - TarBase | |||||
| miR-targeted genes in epithelium - TarBaseWP2770:Reversible Hydration of Carbon Dioxide | |||||
| Uptake of Carbon Dioxide and Release of Oxygen by Erythrocytes | |||||
| Uptake of Oxygen and Release of Carbon Dioxide by Erythrocytes | |||||
| References | |||||
| REF 1 | Bioorg Med Chem. 2009 Oct 15;17(20):7093-9. Epub 2009 Sep 6.Carbonic anhydrase inhibitors. Diazenylbenzenesulfonamides are potent and selective inhibitors of the tumor-associated isozymes IX and XIIover the cytosolic isoforms I and II. | ||||
| REF 2 | Bioorg Med Chem Lett. 2009 Sep 1;19(17):4929-32. Epub 2009 Jul 22.Carbonic anhydrase inhibitors. Inhibition of the Rv1284 and Rv3273 beta-carbonic anhydrases from Mycobacterium tuberculosis with diazenylbenzenesulfonamides. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

