Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0M0WV
|
||||
| Former ID |
DIB002820
|
||||
| Drug Name |
Fasiglifam hemihydrate
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Type 2 diabetes [ICD9: 250; ICD10:E11] | Phase 3 | [524575] | ||
| Company |
Apollo Scientific
|
||||
| Structure |
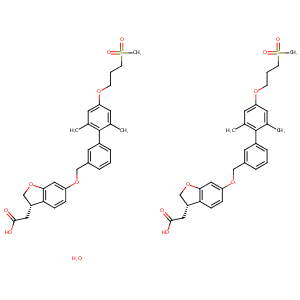
|
Download2D MOL |
|||
| Formula |
C58H66O15S2
|
||||
| Canonical SMILES |
CC1=CC(=CC(=C1C2=CC(=CC=C2)COC3=CC4=C(C=C3)C(CO4)CC(=O)<br />O)C)OCCCS(=O)(=O)C.CC1=CC(=CC(=C1C2=CC(=CC=C2)COC3=CC4=<br />C(C=C3)C(CO4)CC(=O)O)C)OCCCS(=O)(=O)C.O
|
||||
| InChI |
1S/2C29H32O7S.H2O/c2*1-19-12-25(34-10-5-11-37(3,32)33)13-20(2)29(19)22-7-4-6-21(14-22)17-35-24-8-9-26-23(15-28(30)31)18-36-27(26)16-24;/h2*4,6-9,12-14,16,23H,5,10-11,15,17-18H2,1-3H3,(H,30,31);1H2/t2*23-;/m11./s1
|
||||
| InChIKey |
OJXYMYYDAVXPIK-IWKNALKQSA-N
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| Target and Pathway | |||||
| Target(s) | GPR40 | Target Info | Modulator | [532423], [532513] | |
| KEGG Pathway | Insulin secretion | ||||
| References | |||||
| Ref 532423 | TAK-875, a GPR40/FFAR1 agonist, in combination with metformin prevents progression of diabetes and beta-cell dysfunction in Zucker diabetic fatty rats. Br J Pharmacol. 2013 Oct;170(3):568-80. | ||||
| Ref 532513 | A novel antidiabetic drug, fasiglifam/TAK-875, acts as an ago-allosteric modulator of FFAR1. PLoS One. 2013 Oct 10;8(10):e76280. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

