Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0M4WA
|
||||
| Former ID |
DIB018023
|
||||
| Drug Name |
obeticholic acid
|
||||
| Synonyms |
INT747; INT-747; 6-ethylchenodeoxycholic acid; 6-ECDCA; OCA; 6-Et CDCA
|
||||
| Drug Type |
Small molecular drug
|
||||
| Company |
Intercept
|
||||
| Structure |
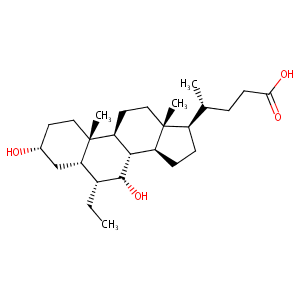
|
Download2D MOL |
|||
| Formula |
C26H44O4
|
||||
| InChI |
InChI=1S/C26H44O4/c1-5-17-21-14-16(27)10-12-26(21,4)20-11-13-25(3)18(15(2)6-9-22(28)29)7-8-19(25)23(20)24(17)30/h15-21,23-24,27,30H,5-14H2,1-4H3,(H,28,29)/t15-,16-,17-,18-,19+,20+,21+,23+,24-,25-,26-/m1/s1
|
||||
| InChIKey |
ZXERDUOLZKYMJM-ZWECCWDJSA-N
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
828938, 828951, 7886586, 7888489, 10300105, 14880306, 16101459, 17396626, 36889785, 46392814, 49700782, 53789454, 57404826, 76486275, 96026040, 103700141, 104638392, 123055342, 126680398, 135263637, 163122223, 175265866, 176236560, 178100437, 204383201, 219813737, 223670674, 224721644, 227005340, 251970994, 252300974, 252553933
|
||||
| Target and Pathway | |||||
| Target(s) | Bile acid receptor | Target Info | Modulator | [889440] | |
| KEGG Pathway | Bile secretion | ||||
| Pathway Interaction Database | RXR and RAR heterodimerization with other nuclear receptor | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

