Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0M6BA
|
||||
| Former ID |
DNCL002406
|
||||
| Drug Name |
AR-67
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Glioblastoma multiforme; Myelodysplastic syndrome [ICD9: 191, 238.7; ICD10:C71, D46] | Phase 2 | [1] | ||
| Company |
Arno Therapeutics
|
||||
| Structure |
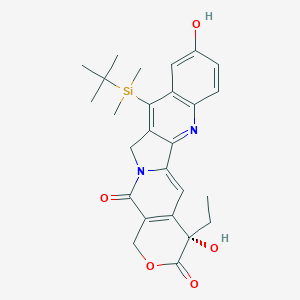
|
Download2D MOL |
|||
| Formula |
C26H30N2O5Si
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Toposisomerase-1 | Target Info | Inhibitor | [2] | |
| NetPath Pathway | IL2 Signaling Pathway | ||||
| PANTHER Pathway | DNA replication | ||||
| Pathway Interaction Database | Caspase Cascade in Apoptosis | ||||
| WikiPathways | Integrated Pancreatic Cancer Pathway | ||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT01124539) Study of AR-67 in Adult Patients With Recurrence of Glioblastoma Multiforme (GBM) or Gliosarcoma. U.S. National Institutes of Health. | ||||
| REF 2 | Protracted dosing of the lipophilic camptothecin analogue AR-67 in non-small cell lung cancer xenografts and humans. Cancer Chemother Pharmacol. 2014 Jul;74(1):45-54. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

