Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0M7PU
|
||||
| Former ID |
DNCL002610
|
||||
| Drug Name |
PM-00104
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Solid tumours [ICD9: 140-199, 210-229; ICD10:C00-D48] | Phase 2 | [1] | ||
| Company |
PharmaMar
|
||||
| Structure |
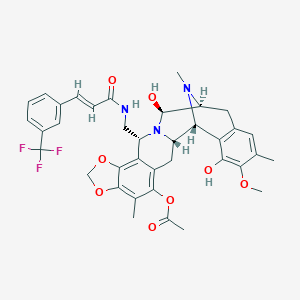
|
Download2D MOL |
|||
| Formula |
C37H38F3N3O8
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Human DNA | Target Info | Modulator | [2] | |
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT00900562) Clinical Trial of PM00104 (Zalypsis) in Patients With Advanced and/or Metastatic Endometrial or Cervical Cancer Previously Treated With One Line of Systemic Chemotherapy. U.S. National Institutes of Health. | ||||
| REF 2 | Zalypsis has in vitro activity in acute myeloid blasts and leukemic progenitor cells through the induction of a DNA damage response. Haematologica. 2011 May;96(5):687-95. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

