Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0MB8I
|
||||
| Former ID |
DAP000440
|
||||
| Drug Name |
Oxacillin
|
||||
| Synonyms |
Bactocill; Ossacillina; Oxacilina; Oxacilline; Oxacillinum; Oxazocillin; Oxazocilline; Prostaphlin; Prostaphlyn; OXACILLIN SODIUM; Ossacillina [DCIT]; Sodium oxacillin; Bactocill (TN); MPI-penicillin; MPi-PC; Oxacilina (TN); Oxacilina [INN-Spanish]; Oxacillin (INN); Oxacillin [INN:BAN]; Oxacilline [INN-French]; Oxacillinum [INN-Latin]; Penicillin, Methylphenylisoxazolyl; Oxacillin, Monosodium Salt, Anhydrous; (2S,5R,6R)-3,3-dimethyl-6-[(5-methyl-3-phenyl-1,2-oxazole-4-carbonyl)amino]-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; (2S,5R,6R)-3,3-dimethyl-6-{[(5-methyl-3-phenylisoxazol-4-yl)carbonyl]amino}-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; (5-methyl-3-phenyl-4-isoxazolyl)penicillin; 2,2-dimethyl-6beta-(5-methyl-3-phenyl-1,2-oxazole-4-carboxamido)penam-3alpha-carboxylic acid; 5-Methyl-3-phenyl-4-isoxazolyl-penicillin; 6beta-(5-methyl-3-phenylisoxazol-4-yl)penicillanic acid
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Bacterial infections [ICD9: 001-009, 010-018, 020-027, 030-041, 080-088, 090-099, 100-104; ICD10:A00-B99] | Approved | [1] | ||
| Therapeutic Class |
Antibiotics
|
||||
| Company |
Sandoz Inc
|
||||
| Structure |
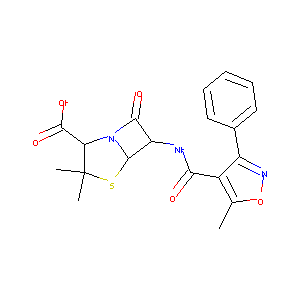
|
Download2D MOL |
|||
| Formula |
C19H19N3O5S
|
||||
| Canonical SMILES |
CC1=C(C(=NO1)C2=CC=CC=C2)C(=O)NC3C4N(C3=O)C(C(S4)(C)C)C<br />(=O)O
|
||||
| InChI |
1S/C19H19N3O5S/c1-9-11(12(21-27-9)10-7-5-4-6-8-10)15(23)20-13-16(24)22-14(18(25)26)19(2,3)28-17(13)22/h4-8,13-14,17H,1-3H3,(H,20,23)(H,25,26)/t13-,14+,17-/m1/s1
|
||||
| InChIKey |
UWYHMGVUTGAWSP-JKIFEVAISA-N
|
||||
| CAS Number |
CAS 66-79-5
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9541, 637164, 7980211, 8153886, 24398251, 29225194, 46505710, 48416356, 50050738, 57323249, 77266000, 96024994, 103215636, 104133798, 104311373, 124766053, 129432415, 134223022, 134337736, 134972946, 136357169, 137003784, 139694323, 144224285, 160964057, 175266996, 175443951, 177749334, 179296061, 223555599, 226395922, 250135015, 252553436
|
||||
| ChEBI ID |
ChEBI:7809
|
||||
| SuperDrug ATC ID |
J01CF04
|
||||
| SuperDrug CAS ID |
cas=000066795
|
||||
| Target and Pathway | |||||
| Target(s) | DNA | Target Info | Binder | [2] | |
| References | |||||
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 061490. | ||||
| REF 2 | Novel anion liposome-encapsulated antisense oligonucleotide restores susceptibility of methicillin-resistant Staphylococcus aureus and rescues mice from lethal sepsis by targeting mecA. Antimicrob Agents Chemother. 2009 Jul;53(7):2871-8. Epub 2009 May 11. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

