Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0N8DP
|
||||
| Former ID |
DIB002497
|
||||
| Drug Name |
Bentazepam
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Anxiety disorder [ICD9: 300, 311; ICD10:F32, F40-F42] | Approved | [551871] | ||
| Structure |
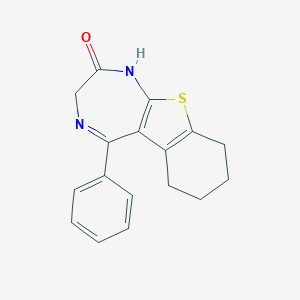
|
Download2D MOL |
|||
| Formula |
C17H16N2OS
|
||||
| Canonical SMILES |
O=C1CN=C(c2ccccc2)c3c4CCCCc4sc3N1
|
||||
| InChI |
1S/C17H16N2OS/c20-14-10-18-16(11-6-2-1-3-7-11)15-12-8-4-5-9-13(12)21-17(15)19-14/h1-3,6-7H,4-5,8-10H2,(H,19,20)
|
||||
| InChIKey |
AIZFEOPQVZBNGH-UHFFFAOYSA-N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | GABA A receptor | Target Info | Modulator | [530960], [551871] | |
| References | |||||
| Ref 530960 | Prodynorphin gene deletion increased anxiety-like behaviours, impaired the anxiolytic effect of bromazepam and altered GABAA receptor subunits gene expression in the amygdala. J Psychopharmacol. 2011Jan;25(1):87-96. | ||||
| Ref 551871 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

