Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0O6SX
|
||||
| Former ID |
DAP000324
|
||||
| Drug Name |
Hydroxyzine
|
||||
| Synonyms |
Atara; Atarax; Ataraxoid; Atarazoid; Atarox; Atazina; Aterax; Deinait; Durrax; Fenarol; Hidroxizina; Hychotine; Hydroksyzyny; Hydroxine; Hydroxizine; Hydroxizinum; Hydroxycine; Hydroxyzin; Hydroxyzinum; Hydroxyzyne; Idrossizina; Neurozina; Nevrolaks; Orgatrax; Pamazone; Parenteral; Paxistil; Placidol; Plaxidol; Tranquizine; Traquizine; Vistaril; Atarax base; Hydroksyzyny [Polish]; Hydroxyzine Hcl; Hydroxyzine base; Hydroxyzine hydrochloride; Idrossizina [DCIT]; NP 212; UCB 4492; UCB 492; Atarax (TN); Hidroxizina [INN-Spanish]; Hy-Pam 25; Hydroxyzine (INN); Hydroxyzine [INN:BAN]; Hydroxyzinum [INN-Latin]; Marex (TN); Neo-Calma; Tran-Q; U.CB 4492; Vesparaz-Wirkstoff; Vistaril (TN); U.C.B 4492; N-(4-Chlorobenzhydryl)-N'-(hydroxyethoxyethyl)piperazine; N-(4-Chlorobenzhydryl)-N'-(hydroxyethyloxyethyl)piperazine; Ethanol, 2-(2-(4-((4-chlorophenyl)phenylmethyl)-1-piperazinyl)ethoxy)-(9CI); 1-(p-Chloro-.alpha.-phenylbenzyl)-4-[2-[(2-hydroxyethoxy)ethyl]piperazine; 1-(p-Chloro-alpha-phenylbenzyl)-4-(2-(2-hydroxyethoxy)ethyl)piperazine; 1-(p-Chloro-alpha-phenylbenzyl)-4-(2-hydroxyethoxyethyl)piperazine; 1-(p-Chlorobenzhydryl)-4-(2-(2-hydroxyethoxy)ethyl)diethylenediamine; 1-(p-Chlorobenzhydryl)-4-(2-(2-hydroxyethoxy)ethyl)piperazine; 1-(p-Chlorobenzhydryl)-4-[2-(2-hydroxyethoxy)ethyl]diethylenediamine; 1-(p-Chlorobenzhydryl)-4-[2-(2-hydroxyethoxy)ethyl]piperazine; 1-(p-Chlorodiphenylmethyl)-4-(2-(2-hydroxyethoxy)ethyl)piperazine; 1-(p-Chlorodiphenylmethyl)-4-[2-(2-hydroxyethoxy)ethyl]piperazine; 2-(2-(4-((4-Chlorophenyl)phenylmethyl)-1-piperazinyl)ethoxy)ethanol; 2-(2-(4-(p-Chloro-alpha-phenylbenzyl)-1-piperazinyl)ethoxy)ethanol; 2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-1-yl}ethoxy)ethanol; 2-[(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-1-yl}ethyl)oxy]ethanol; 2-[2-[4-(p-Chloro-.alpha.-phenylbenzyl)-1-piperazinyl]ethoxy]ethanol; 2-[2-[4-[(4-chlorophenyl)-phenylmethyl]piperazin-1-yl]ethoxy]ethanol
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Hypnotics and Sedatives
|
||||
| Company |
Pfizer Pharmaceuticals
|
||||
| Structure |
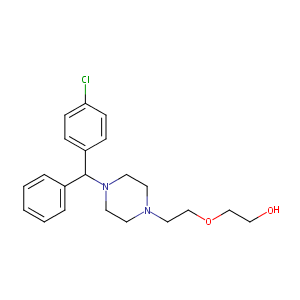
|
Download2D MOL |
|||
| Formula |
C21H27ClN2O2
|
||||
| InChI |
InChI=1S/C21H27ClN2O2/c22-20-8-6-19(7-9-20)21(18-4-2-1-3-5-18)24-12-10-23(11-13-24)14-16-26-17-15-25/h1-9,21,25H,10-17H2
|
||||
| InChIKey |
ZQDWXGKKHFNSQK-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 68-88-2
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9257, 442157, 6240382, 7979552, 8152312, 10532787, 11466161, 11467281, 11485881, 14853297, 29222782, 46508556, 47434366, 47509553, 47583264, 47879646, 48416096, 49698864, 49846689, 49967650, 50139270, 50826383, 57321926, 85209633, 85787868, 85789191, 92729849, 96024747, 103234893, 104304148, 107881553, 118261336, 124561307, 124883569, 124883570, 124883571, 124883573, 124889816, 125823731, 125948273, 126687301, 127310111, 127310112, 127310113, 127310114, 127310115, 129939125, 134337636, 134357363, 134971373
|
||||
| ChEBI ID |
ChEBI:5818
|
||||
| SuperDrug ATC ID |
N05BB01
|
||||
| SuperDrug CAS ID |
cas=000068882
|
||||
| Target and Pathway | |||||
| Target(s) | Histamine H1 receptor | Target Info | Antagonist | [536846], [536963] | |
| PANTHER Pathway | Histamine H1 receptor mediated signaling pathway | ||||
| References | |||||
| Ref 538358 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 085551. | ||||
| Ref 542211 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7199). | ||||
| Ref 536846 | Physicochemical, pharmacological and pharmacokinetic properties of the zwitterionic antihistamines cetirizine and levocetirizine. Curr Med Chem. 2008;15(21):2173-91. | ||||
| Ref 536963 | Hydroxyzine, a first generation H(1)-receptor antagonist, inhibits human ether-a-go-go-related gene (HERG) current and causes syncope in a patient with the HERG mutation. J Pharmacol Sci. 2008 Dec;108(4):462-71. Epub 2008 Dec 5. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

