Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0P2GK
|
||||
| Former ID |
DIB012588
|
||||
| Drug Name |
Sodium phenylbutyrate
|
||||
| Synonyms |
Ammonaps; Buphenyl; EL-532; Sodium 4-phenylbutyrate; Sodium phenylbutyrate, Elan; Sodium phenylbutyrate, Medicis; Sodium phenylbutyrate, Ucyclyd; VP-101; Sodium 4-phenylbutyrate, Elan; Sodium 4-phenylbutyrate, Ucyclyd; Sodium phenylbutyrate (cancer), MacroChem/Access; Sodium phenylbutyrate (cancer), Virium/ Somanta; Sodium phenylbutyrate (cancer), Virium/Access Pharmaceuticals; Sodium 4-phenyibutyrate (cancer), MacroChem/Access; Sodium 4-phenylbutyrate (cancer), Virium/ Somanta; Sodium 4-phenylbutyrate (cancer), Virium/Access Pharmaceuticals
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Spinal muscular atrophy [ICD9: 335.0-335.1; ICD10:G00-G99] | Approved | [522122] | ||
| Company |
Ucyclyd Pharma Inc; Elan Corp plc
|
||||
| Structure |
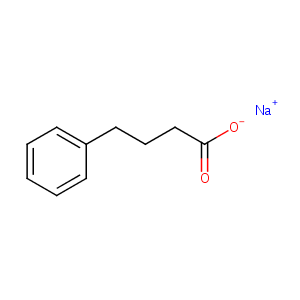
|
Download2D MOL |
|||
| Formula |
C10H11NaO2
|
||||
| Canonical SMILES |
c1(CCCC(=O)[O-])ccccc1.[Na+]
|
||||
| CAS Number |
CAS 1716-12-7
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Histone deacetylase | Target Info | Inhibitor | [530995], [551871] | |
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

