Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0Q9EV
|
||||
| Drug Name |
lifitegrast
|
||||
| Synonyms |
SAR-1118; SAR-1119; SHP606; SPD606; Xiidra
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Dry eye disease [ICD9: 370.33; ICD10:H16.229] | Approved | [889440] | ||
| Company |
Shire Pharmaceuticals
|
||||
| Structure |
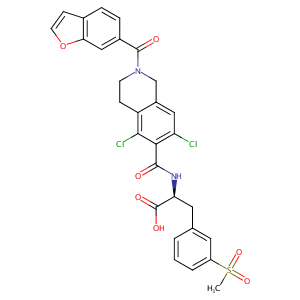
|
Download2D MOL |
|||
| Formula |
C29H24Cl2N2O7S
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Lymphocyte function-associated antigen 1 | Target Info | Modulator | [889440] | |
| KEGG Pathway | Rap1 signaling pathway | ||||
| Cell adhesion molecules (CAMs) | |||||
| Natural killer cell mediated cytotoxicity | |||||
| Leukocyte transendothelial migration | |||||
| Regulation of actin cytoskeleton | |||||
| Malaria | |||||
| Staphylococcus aureus infection | |||||
| HTLV-I infection | |||||
| Epstein-Barr virus infection | |||||
| Rheumatoid arthritis | |||||
| Viral myocarditis | |||||
| NetPath Pathway | TCR Signaling Pathway | ||||
| RANKL Signaling Pathway | |||||
| Pathway Interaction Database | Integrin family cell surface interactions | ||||
| Beta2 integrin cell surface interactions | |||||
| CXCR3-mediated signaling events | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

